
Interpretation: The structures of all the constitutionally isomeric ethers of molecular formula
Concept introduction:
The compounds that have the identical molecular formula but have different connectivity of atoms are termed as constitutional isomers. The constitutional isomers are also known as structural isomers.
The names of the different compounds of ether can be determined by using the IUPAC convention for naming compounds.
Answer to Problem 21P
The structures of all the constitutionally isomeric ethers of molecular formula
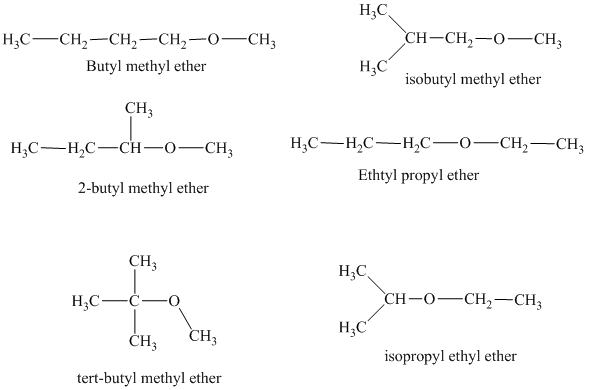
Explanation of Solution
The different constitutionally isomeric ethers that can be written for the given molecular formula, that is,
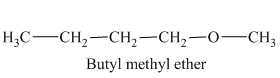
In the above isomeric form, a butyl and a methyl group are attached with the oxygen atom. Therefore, the acceptable name for this isomer is Butyl methyl ether.
Another constitutional isomer for ether of molecular formula
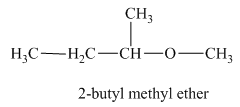
The oxygen atom is attached to the second carbon of the butyl group and a methyl group. Therefore, the acceptable name for this isomer is 2-butyl methyl ether.
Another constitutional isomer for ether of molecular formula
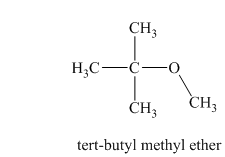
In this isomeric ether, the oxygen atom is attached to a methyl group and a tertiary butyl group. Therefore, the acceptable name for this isomer is tert-butyl methyl ether.
Another constitutional isomer for ether of molecular formula
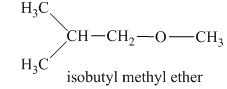
In this isomeric form of ether, the oxygen atom is attached to a methyl group and an isobutyl group. Therefore, the acceptable name for this isomer is isobutyl methyl ether.
Another constitutional isomer for ether of the given molecular formula is shown below.
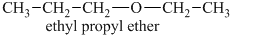
The oxygen atom in the above isomeric form of ether is attached to an ethyl and a propyl group. Therefore, the acceptable name for this isomer is ethyl propyl ether.
Another constitutional isomer for ether of molecular formula
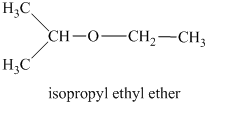
In this isomeric ether, the oxygen atom is attached to an isopropyl and an ethyl group. Therefore, the acceptable name for this isomer is isopropyl ethyl ether.
There are total six constitutional isomers for the ether of molecular formula
Want to see more full solutions like this?
Chapter 17 Solutions
ORGANIC CHEMISTRY VOL 2
- Draw and name all constitutionally isomeric alcohols with the molecular formula C4H10Oarrow_forwardTreatment of alcohol A (molecular formula C5H12O) with CrO3, H2SO4, and H2O affords B with molecular formula C5H10O, which gives an IR absorption at 1718 cm−1. The 1H NMR spectrum of B contains the following signals: 1.10 (doublet, 6 H), 2.14 (singlet, 3 H), and 2.58 (septet, 1 H) ppm. What are the structures of A and B?arrow_forwardCompound A of molecular formula C3H6O shows a noteworthy infrared absorption at 1716 cm-1. Its 1H-NMR spectrum shows one singlet – δ 2.2 (6H) ppm. Its 13C-NMR spectrum has two signals – δ 30, 207 ppm. Suggest a structure for this compound.arrow_forward
- Compound B of molecular formula C9H19N shows a noteworthy infrared absorption at 3300 cm-1. Its 1H-NMR spectrum shows three singlets – δ 1.0 (6H), 1.1 (12H), 1.4 (1H) ppm. Its 13C-NMR spectrum has four signals – δ 25, 28, 41, 64 ppm. Suggest a structure for this compound.arrow_forward(Z)-9-Tricosene [(Z)-CH3(CH2)7CH CH(CH2)12CH3] is the sex pheromone of the female housefly. Synthetic (Z)-9-tricosene is used as bait to lure male flies to traps that contain insecticide. Using acetylene and alcohols of your choice as starting materials, along with any necessary inorganic reagents, show how you could prepare (Z)-9-tricosene.arrow_forwardPropose a structure for an alcohol with molecular formula C5H10O by interpreting the following IR, 1H and 13C NMR. ALSO INDICATE THE FOLLOWING: 1. the name of the compound 2. the condensed structural formula of the compoundarrow_forward
- An unknown compound has a molecular formula of C3H6O2. Its IR spectrum shows a very strong and broad band at 2980 and a strong sharp peak at 1716 cm-1. It exhibits the following signals in its 1H NMR spectrum (ppm): 1.21 (triplet, 3H), 2.48 (quartet, 2H), 11.7 (singlet, 1H); and the following signals in its 13C NMR spectrum (ppm): 8.9, 27.6, 181.5. Draw the structure of the unknown compound.arrow_forwardCompounds B and C are isomers with molecular formula C5H9BrO2. The 1H NMR spectrum of compounds B and C are shown below. The IR spectrum corresponding to compound B showed strong absorption bands at 1739, 1225, and 1158 cm-1, while the spectrum corresponding to compound C have strong bands at 1735, 1237, and 1182 cm-1. 1.Based on the information provided, determine the structure of compounds B and C. 2.Assign all peaks in 1H NMR spectrum of compounds B and C.arrow_forwardTreatment of cis-4-bromocyclohexanol with HO– affords compound A and cyclohex-3-en-1-ol. Treatment of trans-4- bromocyclohexanol under the same conditions forms compound B and cyclohex-3-en-1-ol. A and B contain different functional groups and are not isomers of each other. Propose structures for A and B and offer an explanation for their formation.arrow_forward
- An unknown compound A of molecular formula C10H18O reacts with H2SO4 to form two compounds (B and C)of molecular formula C10H16. B and C both react with H2 in the presence of Pd-C to form decalin. Ozonolysis of B forms D, and ozonolysis of C forms a diketone E of molecular formula C10H16O2. Identify the structures of compounds A, B, C, and E.arrow_forwardExplain the following behaviours :(i) Alcohols are more soluble in water than the hydrocarbons of comparable molecular masses.(ii) Ortho-nitrophenol is more acidic than ortho-methoxyphenol.arrow_forwardWhich of the isomeric alcohols having the molecular formula C6H14O are chiral? Which are achiral?arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,

