
Concept explainers
(a)
Interpretation:
The structure of
Concept Introduction:
The molecular formula of organic compound represents the number of bonded atoms with their atomic symbols. The structural formula represents all the bonded atoms with
Answer to Problem 27P
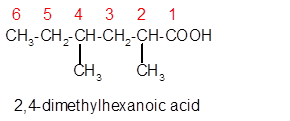
Explanation of Solution
The molecular formula of organic compound represents the number of bonded atoms with their atomic symbols whereas the structural formula represents the arrangement of all the bonded atoms in the molecule.
The carboxylic acid molecular formula
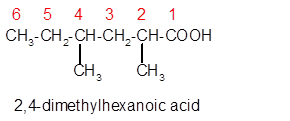
(b)
Interpretation:
The structure of ester molecular formula
Concept Introduction:
The molecular formula of organic compound represents the number of bonded atoms with their atomic symbols. The structural formula represents all the bonded atoms with chemical bonds and the arrangement of atoms in the molecule. The compounds with same molecular formula and different structural formula are known as isomers. Isomers can be classified as constitutional isomers and stereoisomers.
Answer to Problem 27P
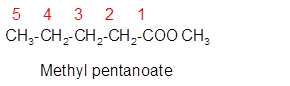
Explanation of Solution
The molecular formula of organic compound represents the number of bonded atoms with their atomic symbols whereas the structural formula represents the arrangement of all the bonded atoms in the molecule.
The ester molecular formula
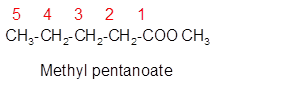
(c)
Interpretation:
The structure of ester having molecular formula
Concept Introduction:
The molecular formula of organic compound represents the number of bonded atoms with their atomic symbols. The structural formula represents all the bonded atoms with chemical bonds and the arrangement of atoms in the molecule. The compounds with same molecular formula and different structural formula are known as isomers. Isomers can be classified as constitutional isomers and stereoisomers.
Answer to Problem 27P
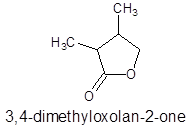
Explanation of Solution
The molecular formula of organic compound represents the number of bonded atoms with their atomic symbols whereas the structural formula represents the arrangement of all the bonded atoms in the molecule.
The ester molecular formula
Want to see more full solutions like this?
Chapter 17 Solutions
General, Organic, and Biological Chemistry - 4th edition
- List the following compounds in order of increasing water solubility: a.ethoxyethane b.propanoic acid c.pentane d.1 butanolarrow_forwardWhat two functional groups react to form the following? a. A hemiacetal b. An acetal c. A ketal d. A hemiketalarrow_forward3-Methyl-2-hexenoic acid (mixture of E and Z isomers) has been identified as the substance responsible for the odor of human sweat. Synthesize the compound from raw materials that have five carbons or less.arrow_forward
- 1. What functional group is produced when an aldehyde reacts with H2/Pt? A.secondary alcohol B. carboxylic acid C.hemiacetal D. primary alcohol E.alkane F.tertiary alcohol G. alkene 2. What reaction occurs when an aldehyde reacts with H2/Pt to form a primary alcohol? A. Hydration B. Hydration C. Dehydration D. Oxidation E. Reduction( hydrogentation) 3. What reaction occurs when an Ester react with H+/H2O to from a carboxylic acid and alcohol? A. Dehydration B. Reduction ( Hydrogenation) C.Hydrolysis D. Hydration E.oxidationarrow_forward14. PART 3: Draw the structure for compound A.arrow_forward1. Draw the organic product formed when the following compounds undergo a substitution reaction ... : (a) acetic acid and methylamine; (b) butanoic acid and 2-propanol; (c) formic acid and 2-methyl- 1-propanol 2. Draw the organic product formed when the following compounds undergo a substitution reaction: (a) acetic acid and 1- hexanol; (b) propanoic acid and dimethylamine; (c) ethanoic acid and diethylamine.arrow_forward
- Which of the following alcohols can be prepared from a Grignard reagent and ethylene oxide? A. only 1 B. only 1 and 2 C. only 1, 2 and 3 D. 1, 2, 3 and 4arrow_forward1-Octen-3-ol is a potent mosquito attractant commonly used in mosquito traps. A number of reactions, including hydrogenation, will transform 1-octen-3-ol into a less effective molecule. Draw the structure of a hydrogenation product of 1-octen-3-ol.arrow_forward1. Carboxylic acid reacts with an alcohol to form: A. Ester and Water B. Ester C. Water D. No reaction 2. The general formula for Carboxylic acids: A. RCOOH B. RCOOR C. RCOR D. RCOH 3. General formula of phenols: * A. ROH B. Ar-OH C. R-SH D. RCOHarrow_forward
- 1. What is the role of the acetic acid in the oxidation of Cyclohexanol to Cyclohexanone? Write the balanced chemical reaction between acetic acid and sodium hypochlorite.2. How do you neutralize the acetic acid regenerated in the reaction? Write the balanced chemical reaction.arrow_forward1)Draw the structure of salicylic acid. Circle the phenol functional group. 2) Aspirin can irritate the stomach. Circle and name the functional group responsible for the irritation of the stomach.arrow_forwardDraw the structure of a molecule that fi ts each description: a. a 2 ° alcohol of molecular formula C 6H 14O b. an ether with molecular formula C 6H 14O that has a methyl group bonded to oxygen c. a 3 ° alkyl halide with molecular formula C 5H 11Brarrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


