
Concept explainers
Consider the following galvanic cell at 25°C:
The overall reaction and equilibrium constant value are
Calculate the cell potential, 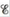 for this galvanic cell and ΔG for the cell reaction at these conditions.
for this galvanic cell and ΔG for the cell reaction at these conditions.
Trending nowThis is a popular solution!

Chapter 17 Solutions
Chemistry: An Atoms First Approach
Additional Science Textbook Solutions
Chemistry: A Molecular Approach
Chemistry & Chemical Reactivity
Introductory Chemistry (6th Edition)
Organic Chemistry (9th Edition)
General, Organic, and Biological Chemistry (3rd Edition)
Living By Chemistry: First Edition Textbook
- An electrolysis experiment is performed to determine the value of the Faraday constant (number of coulombs per mole of electrons). In this experiment, 28.8 g of gold is plated out from a AuCN solution by running an electrolytic cell for two hours with a current of 2.00 A. What is the experimental value obtained for the Faraday Constant?arrow_forwardCalculate the standard cell potential of the cell corresponding to the oxidation of oxalic acid, H2C2O4, by permanganate ion. MnO4. 5H2C2O4(aq)+2MnO4(aq)+6H+(aq)10CO2(g)+2Mn2+(aq)+8H2O(l) See Appendix C for free energies of formation: Gf for H2C2O4(aq) is 698 kJ.arrow_forwardFor each reaction listed, determine its standard cell potential at 25 C and whether the reaction is spontaneous at standard conditions. (a) Mn(s)+Ni2+(aq)Mn2+(aq)+Ni(s) (b) 3Cu2+(aq)+2Al(s)2Al3+(aq)+3Cu(s) (c) Na(s)+LiNO3(aq)NaNO3(aq)+Li(s) (d) Ca(NO3)2(aq)+Ba(s)Ba(NO3)2(aq)+Ca(s)arrow_forward
- Assume the following electrochemical cell simulates the galvanic cell formed by copper and zinc in seawater at pH 7.90 and 25 C. Zn | Zn(OH)2(s) | OH(aq) || Cu(OH)2(s) | Cu(s) a. Write a balanced equation for the reaction that occurs at the cathode. b. Write a balanced equation for the reaction that occurs at the anode. c. Write a balanced chemical equation for the overall reaction. d. Determine the potential (in volts) of the cell.arrow_forwardGiven this reaction, its standard potential, and the standard half-cell potential of 0.34 V for the Cu2+ |Cu half-cell, calculate E° for the Fe(s)|Fe2+(aq) half-cell.arrow_forwardCalculate the cell potential of a cell operating with the following reaction at 25C, in which [MnO4] = 0.010 M, [Br] = 0.010 M. [Mn2] = 0.15 M, and [H] = 1.0 M. 2MNO4(aq)+10Br(aq)+16H+(aq)2MN2(aq)+5Br2(l)+8H2O(l)arrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





