
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 17.9, Problem 17.6P
Interpretation Introduction
Interpretation: It should be accounted for the observation that the given
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
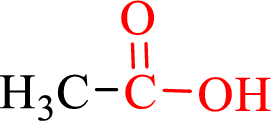
If
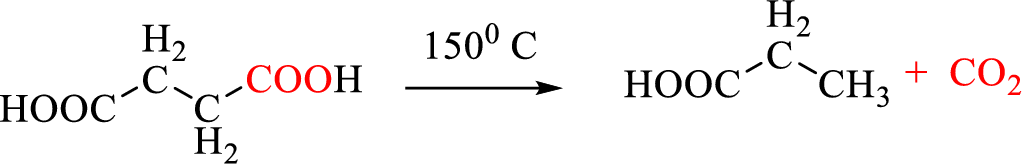
Decarboxylation of
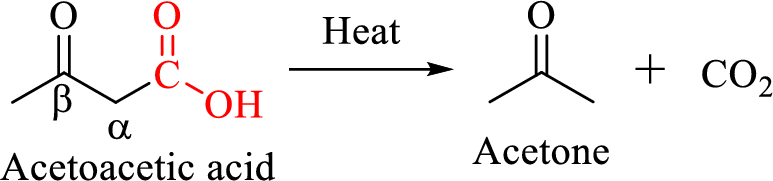
The mechanism of thermal decarboxylation involves two processes,
- (i) Redistribution of electrons in a cyclic transition state.
- (ii) Cyclic transition state possesses keto-enol tautomerism.
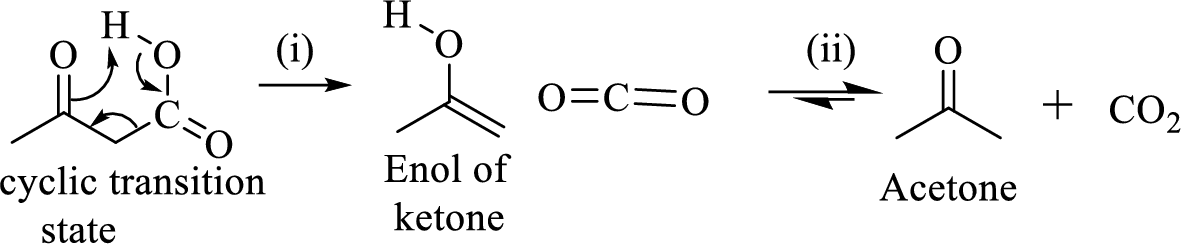
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Treatment of salicylaldehyde (2-hydroxybenzaldehyde) with bromine in glacial acetic acid at 0°C gives a compound with the molecular formula C7H4Br2O2, which is used as a topical fungicide and antibacterial agent. Propose a structural formula for this compound
Draw a line structure clearly showing the stereochemistry of (3S,4S)-4-hydroxy-3,5-dimethylhexanoic acid.
The most stable conformation of most aldopyranoses is one in which the largest group, the CH2OH group, is equatorial. However, alpha-D-idopyranose exists primarily in a conformation with an axial CH2OH group. Write formulas for the two chair conformations of a-D-idopyranose (one with the CH2OH group axial and one with the CH2OH group equatorial) and provide an explanation
Chapter 17 Solutions
Organic Chemistry
Ch. 17.2 - Prob. 17.1PCh. 17.4 - Which is the stronger acid in each pair?Ch. 17.4 - Prob. 17.3PCh. 17.7 - Prob. 17.4PCh. 17.8 - Prob. 17.5PCh. 17.8 - Prob. AQCh. 17.8 - Prob. BQCh. 17.8 - Prob. CQCh. 17.8 - Permethrin and Bifenthrin Pyrethrin is a natural...Ch. 17.9 - Prob. 17.6P
Ch. 17 - Write the IUPAC name of each compound, showing...Ch. 17 - Prob. 17.8PCh. 17 - Prob. 17.9PCh. 17 - Prob. 17.10PCh. 17 - Prob. 17.11PCh. 17 - Prob. 17.12PCh. 17 - Prob. 17.13PCh. 17 - On a cyclohexane ring, an axial carboxyl group has...Ch. 17 - Prob. 17.15PCh. 17 - Prob. 17.16PCh. 17 - Prob. 17.17PCh. 17 - Complete each reaction.Ch. 17 - Prob. 17.19PCh. 17 - Prob. 17.20PCh. 17 - Prob. 17.21PCh. 17 - Show the reagents and experimental conditions...Ch. 17 - Prob. 17.23PCh. 17 - Prob. 17.24PCh. 17 - Prob. 17.25PCh. 17 - In each set, assign the acid its appropriate pKa.Ch. 17 - Low-molecular-weight dicarboxylic acids normally...Ch. 17 - Complete the following acid-base reactions. (a)...Ch. 17 - Prob. 17.29PCh. 17 - Prob. 17.30PCh. 17 - Excess ascorbic acid is excreted in the urine, the...Ch. 17 - Give the expected organic product when...Ch. 17 - Show how to convert trans-3-phenyl-2-propenoic...Ch. 17 - Show how to convert 3-oxobutanoic acid...Ch. 17 - Prob. 17.35PCh. 17 - Prob. 17.36PCh. 17 - Prob. 17.37PCh. 17 - When 4-hydroxybutanoic acid is treated with an...Ch. 17 - Fischer esterification cannot be used to prepare...Ch. 17 - Draw the product formed on thermal decarboxylation...Ch. 17 - Prob. 17.41PCh. 17 - Show how cyclohexanecarboxylic acid could be...Ch. 17 - Prob. 17.43PCh. 17 - Prob. 17.44PCh. 17 - Prob. 17.45PCh. 17 - Write the products of the following sequences of...Ch. 17 - Using your reaction roadmaps as a guide, show how...Ch. 17 - Using your reaction roadmaps as a guide, show how...Ch. 17 - Using your reaction roadmaps as a guide, show how...Ch. 17 - Using your reaction roadmaps as a guide, show how...Ch. 17 - Prob. 17.51PCh. 17 - Complete the following Fischer esterification...Ch. 17 - Prob. 17.53P
Knowledge Booster
Similar questions
- Molecules of 6,6-dinitrobiphenyl-2,2-dicarboxylic acid have no tetrahedral chiral center, and yet they can be resolved to a pair of enantiomers. Account for this chirality.arrow_forwardAlthough ibuprofen is sold as a racemic mixture, only the S enantiomer acts as an analgesic. In the body, however, some of the R enantiomer is converted to the S isomer by tautomerization to an enol and then protonation to regenerate the carbonyl compound. Write a stepwise mechanism for this isomerization.arrow_forwardDraw the structure of sodium tetradecyl sulfate and ciprofloxacin and state how the activity of the drug is influenced by alkane groupings.arrow_forward
- A strong acid is generally used to catalyze the Fischer esterification of carboxylic acids.What two steps in the reaction are accelerated by the presence of strong acid? What function does the acid play in each of these steps?arrow_forwardMyo-inositol, the most prominent naturally occurring form of inositols, is a carbocyclic polyol that plays an important role as the structural basis for a number of secondary messengers in eukaryotic cells. It is generated in vivo from the aldol cyclization of glucose-6-phosphate to myo-inositol-1-phosphatearrow_forwardDefine the Mechanism - Conversion of Carboxylic Acids to Amides with DCCarrow_forward
- When the gum of the shrub Sterculia setigera is subjected to acidic hydrolysis, one of the water-soluble components of thehydrolysate is found to be tagatose. The following information is known about tagatose:(1) Molecular formula C6H12O6(2) Undergoes mutarotation.(3) Does not react with bromine water.(4) Reduces Tollens reagent to give d-galactonic acid and d-talonic acid.(5) Methylation of tagatose (using excess CH3 I and Ag2O) followed by acidic hydrolysis gives1,3,4,5-tetra-O-methyltagatose.(a) Draw a Fischer projection structure for the open-chain form of tagatose.(b) Draw the most stable conformation of the most stable cyclic hemiacetal form of tagatosearrow_forwardIllustrate the osazone of 1,4-dihydroxy-2-butanonearrow_forwardOne of the following compounds undergoes electrophilic aromatic substitution predominantly at C-3, and one undergoes electrophilic aromatic substitution predominantly at C-4. Which is which?arrow_forward
- A step in a synthesis of PGE1 (prostanglandin E1, alprostadil) is reaction of a trisubstituted cyclohexene with bromine to form a bromolactone. Alprostadil is used as temporary therapy for infants born with congenital heart defects that restrict pulmonary blood flow. It brings about dilation of the ductus arteriosus, which in turn increases blood flow in the lungs and blood oxygenation. Propose a mechanism for formation of This bromolactone, and account for the observed stereochemistry of each substituent on the cyclohexane ring.arrow_forwardTreatment of pentanedioic (glutaric) anhydride with ammonia at elevated temperature leads to a compound of molecular formula C5H7NO2. What is the structure of this product? [Hint: You need to think about the reactivity not only of acid anhydrides but also of amides and carboxylic acids]arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning