
a) Styrene (PhCH=CH2)
Interpretation:
How to prepare styrene from 2-phenylethanol is to be stated.
Concept introduction:
Alcohols undergo dehydration when treated with POCl3 in pyridine to yield
To state:
How to prepare styrene from 2-phenylethanol.
Answer to Problem 47AP
Styrene can be prepared by treating 2-phenylethanol with POCl3 in pyridine.
Explanation of Solution
2-Phenylethanol when treated with POCl3 in pyridine eliminates a molecule of water from the side chain to yield styrene.
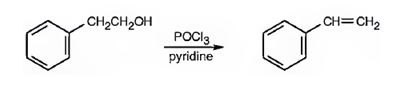
Styrene can be prepared by treating 2-phenylethanol with POCl3 in pyridine.
b) Phenylacetaldehyde (PhCH2CHO)
Interpretation:
How to prepare phenylacetaldehyde from 2-phenylethanol is to be stated.
Concept introduction:
Dess-Martin periodinate in dichloromethane oxidizes 10alcohols to
To state:
How to prepare phenylacetaldehyde from 2-phenylethanol
Answer to Problem 47AP
Phenylacetaldehyde can be prepared by oxidizing 2-phenylethanol with Dess-Martin periodinate in dichloromethane.
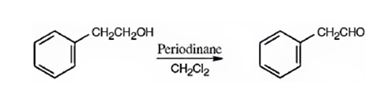
Explanation of Solution
2-phenylethanol is a 10 alcohol. It gets oxidized to phenylacetaldehyde when treated with Dess-Martin periodinate in dichloromethane.
Phenylacetaldehyde can be prepared by oxidizing 2-phenylethanol with Dess-Martin periodinate in dichloromethane.

c) Phenylacetic acid (PhCH2COOH)
Interpretation:
How to prepare phenylacetic acid from 2-phenylethanol is to be stated.
Concept introduction:
CrO3 in acidic solutions oxidize 10 alcohols directly into acids and 20 alcohols to ketones. It does not oxidize 30 alcohols.
To state:
How to prepare phenylacetic acid from 2-phenylethanol.
Answer to Problem 47AP
Phenylacetic acid can be prepared by oxidizing 2-phenylethanol with CrO3 in acidic solutions.
Explanation of Solution
2-phenylethanol is a 10 alcohol. It gets oxidized to phenylacetic acid when treated with CrO3 in acidic solutions.
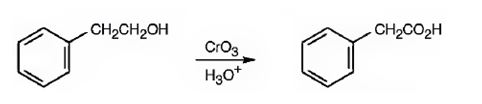
Phenylacetic acid can be prepared by oxidizing 2-phenylethanol with CrO3 in acidic solutions.
d) Benzoic acid
Interpretation:
How to prepare benzoic acid from 2-phenylethanol is to be stated.
Concept introduction:
KMnO4 in acidic solutions oxidize
To state:
How to prepare benzoic acid from 2-phenylethanol.
Answer to Problem 47AP
Benzoic acid can be prepared by oxidizing 2-phenylethanol with KMnO4 in acidic solutions.
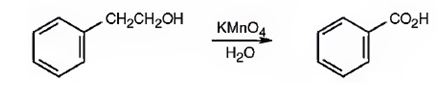
Explanation of Solution
2-phenylethanol is a 10 alcohol. It gets oxidized to benzoic acid when treated with KMnO4 in acidic solutions.
Benzoic acid can be prepared by oxidizing 2-phenylethanol with KMnO4 in acidic solutions.
e) Ethylbenzene
Interpretation:
How to prepare ethylbenzene from 2-phenylethanol is to be stated.
Concept introduction:
Alcohols undergo dehydration when treated with POCl3 in pyridine to yield an alkene. The alkene upon reduction gives the
To state:
How to prepare ethylbenzene from 2-phenylethanol.
Answer to Problem 47AP
Ethylbenzene can be prepared from 2-phenylethanol by following the steps shown below.
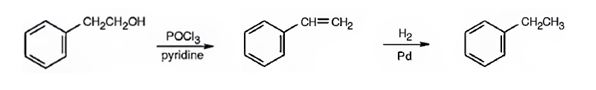
Explanation of Solution
2-Phenylethanol when treated with POCl3 in pyridine eliminates a molecule of water from the side chain to yield styrene. When treated with H2/Pd, the double bond in the side chain gets reduced to yield ethyl benzene.
Ethylbenzene can be prepared from 2-phenylethanol by following the steps shown below.

f) benzaldehyde
Interpretation:
How to prepare benzaldehyde from 2-phenylethanol is to be stated.
Concept introduction:
Alcohols undergo dehydration when treated with POCl3 in pyridine to yield an alkene. The alkene upon ozonolyzis followed by reduction will yield the aldehyde required.
To state:
How to prepare benzaldehyde from 2-phenylethanol.
Answer to Problem 47AP
Benzaldehyde can be prepared from 2-phenylethanol by following the steps shown below.
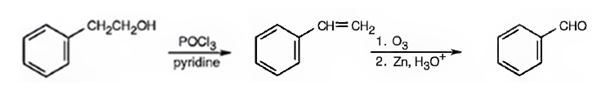
Explanation of Solution
2-Phenylethanol when treated with POCl3 in pyridine eliminates a molecule of water from the side chain to yield styrene. When styrene is subjected to ozonolysis followed by reduction, the double bond in side chain gets cleaved resulting in the formation of benzaldehyde.
Benzaldehyde can be prepared from 2-phenylethanol by following the steps shown below.

g) 1-phenylethanol
Interpretation:
How to prepare 1-phenylethanol from 2-phenylethanol is to be stated.
Concept introduction:
Alcohols undergo dehydration when treated with POCl3 in pyridine to yield an alkene. The alkene adds a molecule of water following oxymercuration-demercuration process. The addition will take place following Markovnikov regiochemistry.
To state:
How to prepare 1-phenylethanol from 2-phenylethanol.
Answer to Problem 47AP
1- Phenylethanol can be prepared from 2-phenylethanol by following the steps shown below.
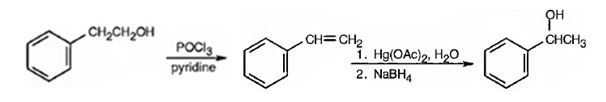
Explanation of Solution
2-Phenylethanol, when treated with POCl3 in pyridine eliminates a molecule of water from the side chain to yield styrene. When styrene is subjected oxymercuration-demercuration processes, a molecule of water is added, following Markovnikov regiochemistry, to the double bond. The –OH adds on to the more alkyl substituted carbon and H to the less alkyl substituted carbon in double bond to yield 1-phenylethanol.
1-Phenylethanol can be prepared from 2-phenylethanol by following the steps shown below.

h) 1-Bromo-2-phenylethane
Interpretation:
How to prepare 1-bromo-2-phenylethane from 2-phenylethanol is to be stated.
Concept introduction:
Alcohols yield the corresponding alkyl bromides when treated with PBr3.
To state:
How to prepare 1-bromo-2-phenylethane from 2-phenylethanol is to be stated.
Answer to Problem 47AP
1-Bromo-2-phenylethane can be prepared from 2-phenylethanol by following the steps shown below.
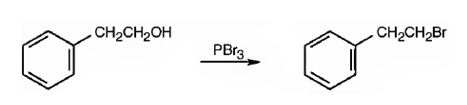
Explanation of Solution
When 2-phenylethanol is treated with PBr3, a bimolecular nucleophilic substitution of –OH by Br takes place to yield 1-bromo-2-phenylethane.
1-Bromo-2-phenylethane can be prepared from 2-phenylethanol by following the steps shown below.

Want to see more full solutions like this?
Chapter 17 Solutions
Organic Chemistry
- Treatment of 1-aminoadamantane, C10H17N, with methyl 2,4-dibromobutanoate in the presence of a nonnucleophilic base, R3N, involves two successive SN2 reactions and gives compound A. Propose a structural formula for compound A.arrow_forwardAmines are converted into alkenes by a two-step process called Hofmann elimination. SN2 reaction of the amine with an excess of CH3I in the first step yields an intermediate that undergoes E2 reaction when treated with silver oxide as base. Pentylamine, for example, yields 1-pentene. Propose a structure for the intermediate, and explain why it readily undergoes elimination.arrow_forward8.b)Provide mechanisms for the following reactions:arrow_forward
- a)Write the SN2 reaction mechanism between iodobutane with sodium hydroxide, NaOH. b)Predict the products and show the SN1 reaction mechanism that occurs with 2-iodo-2- methylpropane in aqueous sodium hydroxide, NaOH.arrow_forward(a) Arrange the following compounds in an increasing order of their indicated property :(i) Benzoic acid, 4-Nitrobenzoic acid, 3,4-Dinitrobenzoic acid, 4-Methoxybenzoic acid (acid strength)(ii) CH3CH2CH (Br) COOH, CH3CH (Br) CH2COOH,(CH3)2CHCOOH, CH3CH2CH2COOH (acid strength)(b) How would you bring about the following conversions :(i) Propanone to Propene (ii) Benzoic acid to Benzaldehyde(iii) Bromobenzene to 1-phenylethanolarrow_forwardGive reagents necessary and show isolated intermediates for the synthesis below.arrow_forward
- How do you synthesize 3-oxocyclohexanecarboxylic acid from 2-cyclohexenone?give earrow_forwardReaction of N,N-diethyl-p-diaminobenzene with sodium nitrite and hydrochloric acid at 0°C, followed by treatment with nitrobenzene.arrow_forwardHow would you prepare the following compounds from benzene, using a diazonium replacement reaction in your scheme? (a) p-Bromobenzoic acid (b) m-Bromobenzoic acid (c) m-Bromochlorobenzene (d) p-Methylbenzoic acid (e) 1, 2, 4-Tribromobenzenearrow_forward
- A step in a synthesis of PGE1 (prostaglandin E1, alprostadil) is the reaction of a trisubstituted cyclohexene with bromine to form a bromolactone. Propose a mechanism for formation of this bromolactone and account for the observed stereochemistry of each substituent on the cyclohexane ring. Alprostadil is used as a temporary therapy for infants born with congenital heart defects that restrict pulmonary blood flow. It brings about dilation of the ductus arteriosus, which in turn increases blood flow in the lungs and blood oxygenation.arrow_forwardShow the products you would obtain by acid-catalyzed reaction of cyclohexanone with ethylamine, CH3CH2NH2, and with diethylamine, (CH3CH2) 2NH.arrow_forwardPropose a mechanism for the acid-catalyzed hydration of methylidenecyclohexane to give 1-methylcyclohexanol. Which step in your mechanism is rate-determining?arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

