
Concept explainers
(a)
Interpretation:
The reaction of phenyl acetic acid with given reagents should be completed.
Concept Introduction:
Phenylacetic acid contains one −COOH group with molecular formula C6 H5 -CH2 -COOH. Carboxylic acids react with base to form salt and water. This reaction is called as neutralization reaction. The reduction of
Answer to Problem 18.45P
Explanation of Solution
The reaction of C6 H5 -CH2 -COOH with sodium carbonate forms sodium salt of C6 H5 -CH2 -COOH with water and carbon dioxide gas.
(b)
Interpretation:
The reaction of phenyl acetic acid with given reagents should be completed.
Concept Introduction:
Phenylacetic acid contains one −COOH group with molecular formula C6 H5 -CH2 -COOH. Carboxylic acids react with base to form salt and water. This reaction is called as neutralization reaction. The reduction of carboxylic acid form carbonyl compounds and alcohols whereas reaction with alcohol forms ester.
Answer to Problem 18.45P
Explanation of Solution
The reaction of C6 H5 -CH2 -COOH with sodium hydroxide forms sodium salt of C6 H5 -CH2 -COOH with water. This is a neutralization reaction as carboxylic acid reacts with base to form salt.
(c)
Interpretation:
The reaction of phenyl acetic acid with given reagents should be completed.
Concept Introduction:
Phenylacetic acid contains one −COOH group with molecular formula C6 H5 -CH2 -COOH. Carboxylic acids react with base to form salt and water. This reaction is called as neutralization reaction. The reduction of carboxylic acid form carbonyl compounds and alcohols whereas reaction with alcohol forms ester.
Answer to Problem 18.45P
Explanation of Solution
The reaction of C6 H5 -CH2 -COOH with ammonia solution forms ammonium salt of C6 H5 -CH2 -COOH that is ammonium phenyl acetate.
(d)
Interpretation:
The reaction of phenyl acetic acid with given reagents should be completed.
Concept Introduction:
Phenylacetic acid contains one −COOH group with molecular formula C6 H5 -CH2 -COOH. Carboxylic acids react with base to form salt and water. This reaction is called as neutralization reaction. The reduction of carboxylic acid form carbonyl compounds and alcohols whereas reaction with alcohol forms ester.
Answer to Problem 18.45P
Explanation of Solution
The reaction of C6 H5 -CH2 -COOH with lithium ammonium hydride followed by hydrolysis leads to formation of 2-phenylethanol. This is an example of reduction reaction and LiAlH4 is a reducing agent.
(e)
Interpretation:
The reaction of phenyl acetic acid with given reagents should be completed.
Concept Introduction:
Phenylacetic acid contains one −COOH group with molecular formula C6 H5 -CH2 -COOH. Carboxylic acids react with base to form salt and water. This reaction is called as neutralization reaction. The reduction of carboxylic acid form carbonyl compounds and alcohols whereas reaction with alcohol forms ester.
Answer to Problem 18.45P
C6 H5 -CH2 -COOH + NaBH4 / H2 O (No reaction.
Explanation of Solution
NaBH4 with water is a weak reducing agent therefore it cannot reduce the carboxylic acid and no reaction is observed with it.
(f)
Interpretation:
The reaction of phenyl acetic acid with given reagents should be completed.
Concept Introduction:
Phenylacetic acid contains one −COOH group with molecular formula C6 H5 -CH2 -COOH. Carboxylic acids react with base to form salt and water. This reaction is called as neutralization reaction. The reduction of carboxylic acid form carbonyl compounds and alcohols whereas reaction with alcohol forms ester.
Answer to Problem 18.45P
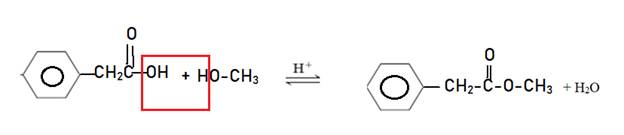
Explanation of Solution
Reaction of phenyl acetic acid with methanol in acidic medium as catalyst forms ester with water. This reaction is called as Fisher Esterification reaction.
(g)
Interpretation:
The reaction of phenyl acetic acid with given reagents should be completed.
Concept Introduction:
Phenylacetic acid contains one −COOH group with molecular formula C6 H5 -CH2 -COOH. Carboxylic acids react with base to form salt and water. This reaction is called as neutralization reaction. The reduction of carboxylic acid form carbonyl compounds and alcohols whereas reaction with alcohol forms ester.
Answer to Problem 18.45P
Explanation of Solution
H2 / Ni cannot reduce carboxylic acid group as it is reducing agent for
Want to see more full solutions like this?
Chapter 18 Solutions
Introduction to General, Organic and Biochemistry
- 18-28 Arrange these compounds in order of increasing acidity: benzoic acid, benzyl alcohol, phenol.arrow_forward18-31 Formic acid is one of the components responsible for the sting of biting ants and is injected under the skin by bee and wasp stings. The pain can be relieved by rubbing the area of the sting with a paste of baking soda (NaHCO3) and water, which neutralizes the acid. Write an equation for this reaction.arrow_forward8 (Chemical Connections 19C) Once it has been opened, and particularly if it has been left open to the air, a bottle of aspirin may develop a vinegar-like odor. Explain how this might happen.arrow_forward
- 2 (Chemical Connections 19A) Locate the ester group in pyrethrin I and draw a structural formula for chrysanthemic acid, the carboxylic acid from which this ester is derived.arrow_forward17-35 Suppose that you take a bottle of benzaldehyde (a liquid, bp 179°C) from a shelf and find a white solid in the bottom of the bottle. The solid turns litmus red; that is, it is acidic. Yet aldehydes are neutral compounds. How can you explain these observations?arrow_forward7 What type of structural feature do the anhydrides of phosphoric acid have in common with carboxylic acids?arrow_forward
- 18-47 Methylparaben and propylparaben are used as preservatives in foods, beverages, and cosmetics. Show how each of these preservatives can be prepared from 4-aminobenzoic acid.arrow_forward16-13 Classify each amino group as primary, secondary, or tertiary and as aliphatic or aromatic. Serotonin (a neurotransmitter) Diphenhydramine (the hydrochloride salt is the antihistamine Benadryl) Lysine (an amino acid)arrow_forward16-26 The p/fb of amphetamine is approximately 3.2 Amphetamine (a) Which form of amphetamine (the free base or its conjugate acid) would you expect to be present at pH 1.0, the pH of stomach acid? (b ) Which form of amphetamine would you expect to be present at pH 7.40, the pH of blood plasma?arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

