
Concept explainers
How many moles of electrons are required to produce (a) 0.84 L of O2 at exactly 1 atm and 25°C from aqueous H2SO4 solution; (b) 1.50 L of Cl2 at 750 mmHg and 20°C from molten NaCl; (c) 6.0 g of Sn from molten SnCl2?
(a)
Interpretation:
Need to calculate the Faraday of electricity needed for the production of 0.84L of O2 with 1 atm pressure upon electrolysis of aqueous H2SO4 at 25oC.
Concept introduction:
Electrolysis of aqueous sulfuric acid i.e. acidified water resulted in the production of oxygen and hydrogen gas, which will be liberated at the anode and cathode respectively. The presence of H+ and SO-4 made the solution to be more conductivity. SO-4 is more stable to be inert at the anode.
Further the cell reaction can be written as shown below
Since volume and pressure of oxygen produced was given, by applying it into ideal gas equation we can calculate the number of mole of oxygen produced
The ideal gas equation can be given as follows.
On applying the number of moles of oxygen produced into stoichiometry of the reaction, the number of moles electron involved in the reaction can be calculated. In case of the given reaction 4 mole of electron was liberated during the production of one mole of oxygen. Since one Faraday is equal to one mole of electron, so 4 Faraday of electricity will be needed to produce one mole of oxygen.
Finally the Faraday of electricity utilized to produce the required amount of oxygen can be calculated according the formula
To find: Amount of Faraday of electricity need to produce 0.076L of O2 with pressure 755mmHg, at 298K, through electrolysis of water.
Answer to Problem 18.52QP
Ideal gas equation can be used to calculate the number of moles of oxygen produced, from that faraday of electricity needed will be calculated in successive steps (a)
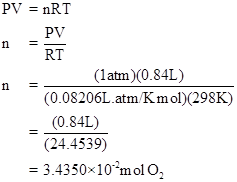
Since one mole of oxygen need 4 Faraday of electricity, so the Faraday of electricity needed to produce
Faraday of electricity need to produce 0.84L of oxygen with pressure 1 atm was calculated as
Explanation of Solution
Ideal gas equation can be used to calculate the number of moles of oxygen produced
Since one mole of oxygen need 4 Faraday of electricity, so the Faraday of electricity needed to produce
Faraday of electricity need to produce 0.84L of oxygen with pressure 1 atm was calculated as
At first the number of moles of oxygen produced through electrolysis was calculated using ideal gas equation, from the given volume and pressure. Thus it was calculated as
The amount of electricity needed to produce 0.84L of oxygen with pressure of 1atm was determined to be
(b)
Interpretation:
Need to calculate the Faraday of electricity needed for the production of 1.50L of Cl2 with pressure 750 mmHg at 20oC by electrolysis of molten NaCl.
Concept introduction:
Electrolysis of molten sodium chloride was represented by the below equation. By using ideal gas equation, the number of moles of chlorine liberated at the anode can be calculated, provided the pressure and volume are known.
The ideal gas equation can be given as follows.
The equation we find that two mole of electron is needed to produce one mole of chlorine gas , Since one Faraday is equal to one mole of electron, The Faraday of electricity utilized to produce given amount of chlorine can be calculated according the formula
To find: Faraday of electricity need to produce 1.50 L of Cl2 with pressure 750mmHg, at 293K, through electrolysis of molten NaCl.
Answer to Problem 18.52QP
Ideal gas equation can be used to calculate the number of moles of chlorine gas produced, Further from the number of moles of chlorine; the faraday of electricity utilized will be calculated in successive steps (b)
Since one mole of chlorine need two Faraday of electricity, so the Faraday of electricity needed to produce
Faraday of electricity need to produce 1.50L of chlorine with pressure 750mm Hg was calculated as
Explanation of Solution
Ideal gas equation can be used to calculate the number of moles of oxygen produced
Since one mole of chlorine need two Faraday of electricity, so the Faraday of electricity needed to produce
The number of moles of chlorine produced through electrolysis was calculated using ideal gas equation, from the given volume and pressure it was calculated as
The amount of electricity needed to produce 1.5 L of chlorine with pressure of 750 mmHg was determined to be
(c)
Interpretation:
Need to calculate the Faraday of electricity needed for the production of 6g of Sn though electrolysis of molten SnCl2.
Concept introduction:
Electrolysis of molten stannous chloride will result in the formation of Tin (Sn) and chlorine gas and half-cell reaction at the anode and cathode was given below. In this two electrons were released by chloride ion at the anode and get liberated as chlorine gas, further stannous ion accept two electron and for tin metal
The ideal gas equation can be given as follows.
Since the mass of the metal produced was given, from that number of moles of Sn can be calculated. Further from the number of moles of Sn produced the Faraday of electricity can be calculated by the formula given below, since one Faraday is equal to one mole of electron. In the present case 2 moles of electrons are needed to reduce one mole of Sn2+
So
To find: Faraday of electricity need to produce 6 g of Tin, by electrolysis of molten SnCl2.
Answer to Problem 18.52QP
From the mass of tin produced during the electrolysis, the Faraday of electricity needed for reaction can be calculated in the following steps (c).
Since one mole of Sn2+ ion need two Faraday of electricity to, so the Faraday of electricity needed to produce
Explanation of Solution
From the molar mass calculation
Weight = 6g
Atomic Mass = 118.7g
Since one mole of Sn2+ ion need two Faraday of electricity, so the Faraday of electricity needed to produce
The number of moles of tin produced through electrolysis was calculated as
The Faraday of electricity need to produce 6g of Tin from by electrolysis of molten SnCl2 was identified as
Want to see more full solutions like this?
Chapter 18 Solutions
Chemistry
- When water is added to a mixture of aluminum metal and sodium hydroxide, hydrogen gas is produced. This is the reaction used in commercial drain cleaners: 2Al(s)+6H2O(l)+2OH(aq)2Al(OH)4(aq)+3H2(g)A sufficient amount of water is added to 49.92 g of NaOH to make 0.600 L of solution; 41.28 g of Al is added to this solution and hydrogen gas is formed. (a) Calculate the molarity of the initial NaOH solution. (b) How many moles of hydrogen were formed? (c) The hydrogen was collected over water at 25C and 758.6 mm Hg. The vapor pressure of water at this temperature is 23.8 mm Hg. What volume of hydrogen was generated?arrow_forwardWhen hydrogen peroxide decomposes, oxygen is produced: 2H2O2(aq)2H2O+O2(g)What volume of oxygen gas at 25C and 1.00 atm is produced from the decomposition of 25.00 mL of a 30.0% (by mass) solution of hydrogen peroxide (d=1.05g/mL)?arrow_forwardInsulin is a hormone responsible for the regulation of glucose levels in the blood. An aqueous solution of insulin has an osmotic pressure of 2.5 mm Hg at 25C. It is prepared by dissolving 0.100 g of insulin in enough water to make 125 mL of solution. What is the molar mass of insulin?arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





