
Interpretation:
The set of equations (or a small program) to evaluate the constant-volume heat capacity for a moleculeis to be stated. The graph of the result is to be plotted. The trend for the same is to be stated. The heat capacity versus temperature (say from
Concept introduction:
The heat capacity at constant volume for nonlinear polyatomic molecule is given by the formula,
Where,
•
•
•
•
Answer to Problem 18.59E
The set of equations (or a small program) to evaluate the constant-volume heat capacity for a molecule are,
•
•
•
•
•
•
•
The plot between
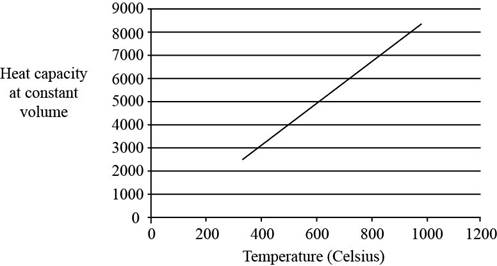
The plot between
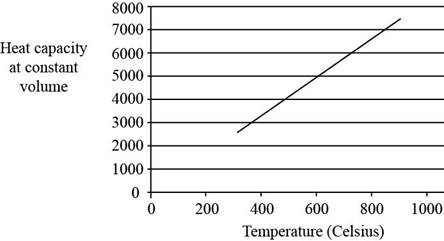
Explanation of Solution
The heat capacity at constant volume for nonlinear polyatomic molecule is given by the formula,
The set of equations(or a small program) to evaluate the constant-volume heat capacity for a molecule are shown below.
•
•
•
•
•
•
•
The vibrational temperatures for
Substitute the value of vibrational temperatures for
The value of
The plot between
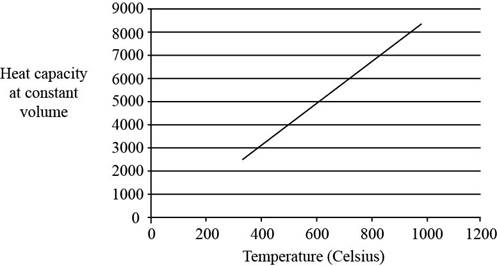
Figure 1
The three vibrational temperatures for
Substitute the value of vibrational temperatures for
The value of
The plot between
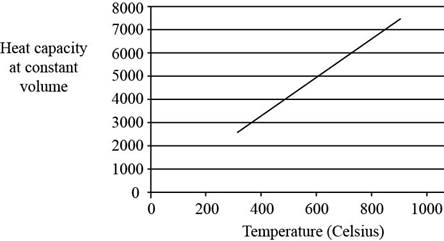
Figure 2
The set of equations (or a small program) to evaluate the constant-volume heat capacity for a molecule are,
•
•
•
•
•
•
•
The plot between
Want to see more full solutions like this?
Chapter 18 Solutions
Physical Chemistry
- What are the numerical values of the heat capacities c-v and c-p of a monatomic ideal gas,in units of cal/mol.K and L.atm/mol.K?arrow_forwardA 1.00 mol sample of H2 is carefully warmed from 22 K to 40 K at constant volume. a What is the expected heat capacity of the hydrogen? b What is q for the process?arrow_forwardThe ground level of Cl is 2P3/2 and a 2P1/2 level lies 881 cm−1 above it. Calculate the electronic contribution to the heat capacity of Cl atoms at (i) 500 K and (ii) 900 K.arrow_forward
- Calculate the theoretical value of C_p (constant pressure heat capacity)/ C_v (constant volume heat capacity) for the following gases: Ar, CO_2, and N_2arrow_forwardWhat is the heat capacity at constant volume of nn mole monatomic gas?arrow_forwardUse the equipartition theorem to estimate the constant- volume molar heat capacity of (i) O3, (ii) C2H6, (iii) CO2 in the gas phase at 25 °C.arrow_forward
- Calculate the molar internal energy of a monatomic gas.arrow_forwardUse the equipartition theorem to estimate the constant-volume molar heat capacity of (i) I2, (ii) CH4, (iii) C6H6 in the gas phase at 25 °C.arrow_forwardCalculate the vibrational, rotational, and translational contributions to the constant volume heat capacity (Cv) for 14N2 at 298 K. Assume this represents the high temperature limit for rotational energy and low temperature limit for vibrational energy. Given that Cv=20.81 J/K·mol for N2, state which type or types of energy contribute most to Cv for N2 and explain why those types of energy contribute most.arrow_forward
- When 178 J of energy is supplied as heat at constant pressure to 1.9 mol of gas molecules, the temperature of the sample increases by 1.78 K. Calculate the molar heat capacities at constant volume and constant pressure of the gas.arrow_forwardA linear molecule may rotate about two axes. If the molecule consists of N atoms, then there are 3N- 5 vibrational modes. Use the equipartition theorem to estimate the total contribution to the molar internal energy from translation, vibration, and rotation for (a) carbon dioxide, CO2, and (b) dibromoethyne, C2Br2, at 2000 K. In contrast, a nonlinear molecule may rotate about three axes and has 3N- 6 vibrational modes. Estimate the total contribution to the molar in ternal energy from translation, vibration, and rotation for (c) nitrogen dioxide, NO2, and (d) tetrabromoethene, C2Br4,at 2000 K. In each case, first assume that all vibrations are active; then assume that none is.arrow_forwardThe specific heat capacities of Li(s), Na(s), K(s), Rb(s),and Cs(s) at 25°C are 3.57, 1.23, 0.756, 0.363, and0.242 J K-1 g-1 , respectively. Compute the molar heatcapacities of these elements and identify any periodictrend. If there is a trend, use it to predict the molar heatcapacity of francium, Fr(s).arrow_forward
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
