
Concept explainers
Interpretation:
A reaction roadmap have to be made for the reactions in the study Guide section of problems 15.19, 16.72 and 17.45.
Concept Introduction:
Addition Reaction: It is defined as
Addition of alcohols to
Example: the reaction of methanol with ethanol to form hemiacetals or acetals.
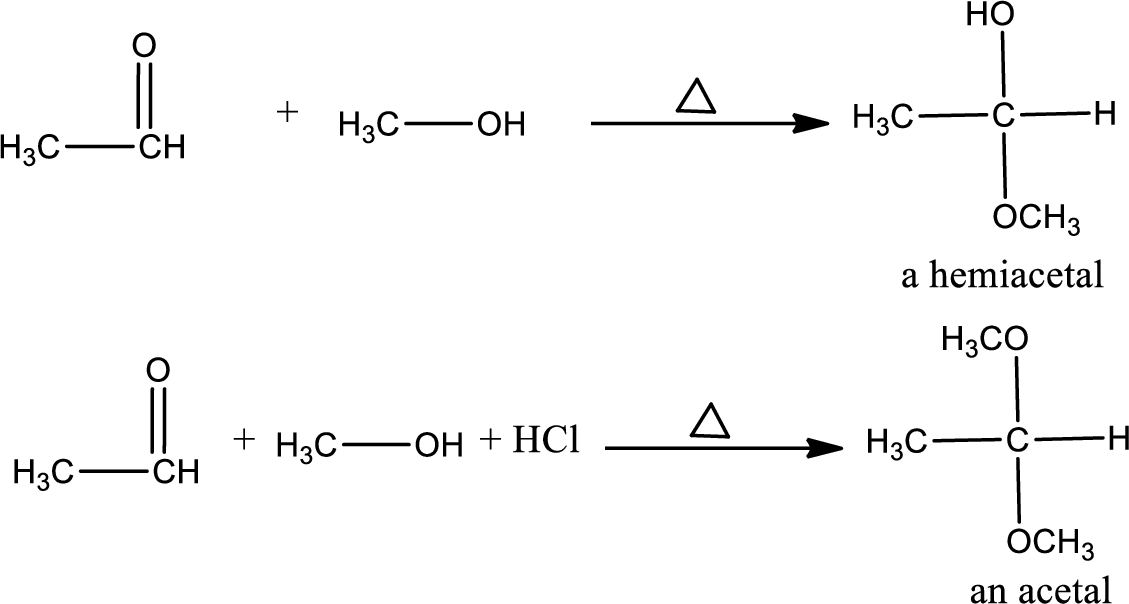
Unsaturated compound: The nucleophile reacts with
The alkylation at the β carbon of ketone or aldehyde is done by the following mechanism;
(1) Alkylating the β carbon via enamine intermediate.
(2) Alkylating the β carbon via a Michael reaction.
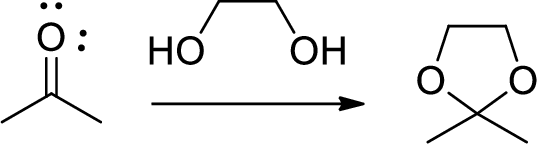
Acetals: Acetals are used to protect the ketone and aldehyde (carbonyl group).
In this reaction acetone is protected as acetal by using ethylene glycol. Acetals are less stable compound.
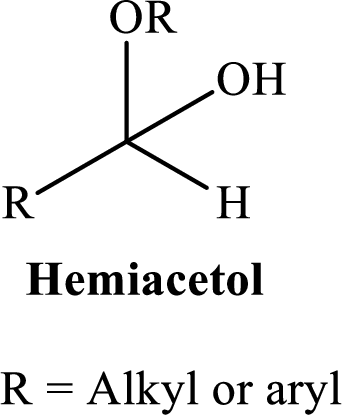
A hemiacetal or a hemiketal: addition of alcohol to an aldehyde or ketone which produce hemi acetal.
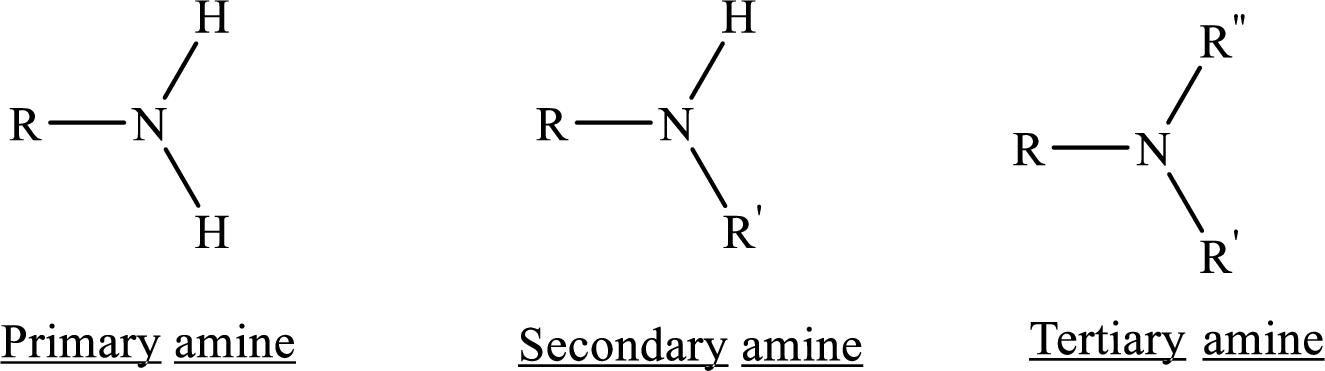
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.
Trending nowThis is a popular solution!

Chapter 18 Solutions
Organic Chemistry
- Write the products of the following sequences of reactions. Refer to your reaction roadmaps to see how the combined reactions allow you to navigate between the different functional groups. Note that you will need your old Chapters 611, Chapters 1518, and Chapter 19 roadmaps along with your new Chapter 20 reaction roadmap for these.arrow_forwardUsing your reaction roadmap as a guide, show how to convert 1-bromopentane and sodium cyanide into N-hexylhexanamide. You must use 1-bromopentane and sodium cyanide as the source of all carbon atoms in the target molecule. Show all reagents and all molecules synthesized along the way.arrow_forwardUsing your reaction roadmap as a guide, show how to convert 2-methylpentane into 2-methyl-3-pentanone. Show all reagents needed and all molecules synthesized along the way.arrow_forward
- Using your reaction roadmaps as a guide, show how to convert propane into propyl propanoate. You must use propane as the source of all carbon atoms in the target molecule. Show all reagents needed and all molecules synthesized along the way.arrow_forwardFill in the appropriate reagents for the following reaction:arrow_forwardWrite the products of the following sequences of reactions. Refer to your reaction roadmaps to see how the combined reactions allow you to navigate between the different functional groups. Note that you will need both your old Chapters 611 roadmap and your new Chapter 15 roadmap for these.arrow_forward
- Using your reaction roadmaps as a guide, show how to convert 4-methyl-1-pentene and carbon dioxide into 5-methylhexanoic acid. You must use 4-methyl-1-pentene and carbon dioxide as the source of all carbon atoms in the target molecule. Show all reagents and all molecules synthesized along the way.arrow_forwardUsing your reaction roadmap as a guide, show how to convert butane into butanal. Show all reagents needed and all molecules synthesized along the way.arrow_forwardUsing your reaction roadmaps as a guide, show how to convert ethanol into 2-pentanone. You must use ethanol as the source of all carbon atoms in the target molecule. Show all reagents and all molecules synthesized along the way.arrow_forward
- Write the products of the following sequences of reactions. Refer to your reaction roadmaps to see how the combined reactions allow you to navigate between the different functional groups. Note that you will need your old Chapters 611, Chapters 1518, and Chapter 19 roadmaps along with your new Chapters 2021 roadmaps for these.arrow_forwardUsing your reaction roadmap as a guide, show how to convert propane into butyronitrile. You must use propane and sodium cyanide as the source of all of the carbon atoms in the butyronitrile product. Show all reagents and all molecules synthesized along the way.arrow_forwardUsing your reaction roadmap as a guide, show how to convert 1-bromopropane and carbon dioxide into 4-heptanone. You must use 1-bromopropane and carbon dioxide as the source of all carbon atoms in the target molecule. Show all reagents and all molecules synthesized along the way.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
