
To determine:
Condensed structural formula for activated lauric acid
Explanation of Solution
The condensed structural formula is an easier way to explain the structure of any molecule as it draws lines between group of atoms attached together and do not draw all the bonds.
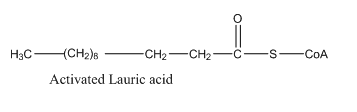
Hence, the condensed structural formula for the activated acrylic acid is given as follows:
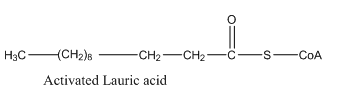
To determine:
Alpha and beta carbon atom in lauroyl- CoA
Explanation of Solution
The alpha and beta positions are determined from adjacent to the functional group. Here, the functional group is carbonyl carbon from where the adjacent carbon will be alpha and next will be beta carbon. So, the
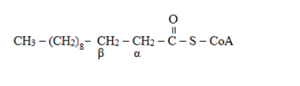
To determine:
Explanation of Solution
The number of beta oxidation cycle for the complete oxidation of lauric acid.
Beta oxidation is a process where the fatty acid is degraded or broken down from its beta carbon position.
Hence, the number of beta oxidation cycles required depends upon the number of carbon atoms present in the acid molecule.
Every two carbon atoms in any acid will produce one acetyl CoA molecule, so when the lauric acid has 12 carbon atoms, it will produce a total of six acetyl CoA molecules.
Further, the number of beta oxidation cycle is considered as one number less than the number of acetyl CoA produced.
Hence, the beta oxidation cycles will be five.
To determine:
Explanation of Solution
The number of acetyl CoA produced from the complete oxidation of lauric acid.
Beta oxidation is a process where the fatty acid is degraded or broken down from its beta carbon position.
Hence, the number of acetyl CoA produced depends upon the number of carbon atoms present in the acid molecule.
Every two carbon atoms in any acid will produce one acetyl CoA molecule, so when the lauric acid has 12 carbon atoms, it will produce a total of six acetyl CoA molecules.
To determine:
Total ATP yield from the given table:

Explanation of Solution
Lauric Acid is a C12 fatty acid and for the activation of lauric acid 2 ATP are required.
The acetyl group of the acetyl CoA is formed by two carbons. And in the last round two acetyl CoA are produced. Accordingly, the number of cycles of ß-oxidation and the number of acetyl CoA produced has been calculated.
From the complete ß-oxidation of lauric acid, total six (6) acetyl CoA, 5 NADH and 6 FADH2 has been produced. Each Acetyl CoA yields 10 ATP, each NADH yields 2.5 ATP and each FADH2 yields 1.5 ATP. Accordingly, ATP yield has been calculated.
Formula used: Number of cycles of ß-oxidation needed for the complete oxidation of fatty acid =
Where n = Number of carbon atoms present in fatty acid.
Number of acetyl CoA produced from the complete oxidation of fatty acid =
Where n = Number of carbon atoms present in fatty acid.
Calculation: Here, number of carbon atoms in the given fatty acid = 12. So, by putting n = 12
Number of cycles of ß-oxidation needed for the complete oxidation of fatty acid =
Therefore, number of acetyl CoA produced from the complete oxidation of fatty acid =
| Activation | -2 ATP | |
| Acetyl CoA | 60ATP | |
| NADH | 12.5 ATP | |
| FADH2 | 7.5 ATP | |
| Total | 78 ATP |
- -carbon atoms in lauroyl-CoA is:
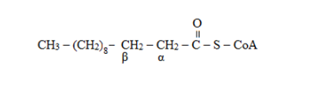
- The number of beta oxidation cycle for complete oxidation of lauric acid will be five.
- The number of acetyl CoA produced from the complete oxidation of lauric acid will be six.
| Activation | -2 ATP | |
| Acetyl CoA | 60ATP | |
| NADH | 12.5 ATP | |
| FADH2 | 7.5 ATP | |
| Total | 78 ATP |
Want to see more full solutions like this?
Chapter 18 Solutions
CHEMISTRY F/RADFORD UNIV.W/MASTERI >LL
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





