
Sketch a galvanic cell, and explain how it works. Look at Figs. 18.1 and 18.5. Explain what is occurring in each container and why the cell in Fig. 18.5 “works,” but the one in Fig. 18.1 does not.
Interpretation: A galvanic cell should be sketched and how it works should be explained. Considering the figures
Concept Introduction:A Galvanic cell also known as electrochemical battery, a device that exploits a spontaneous oxidation reduction reaction to produce electrical energy. Here oxidizing agent and reducing agent has been separated, so that the electron/s generated by oxidation should travel through a wire from reducing agent to oxidizing agent.
Explanation of Solution
The galvanic cell is sketched as follows:
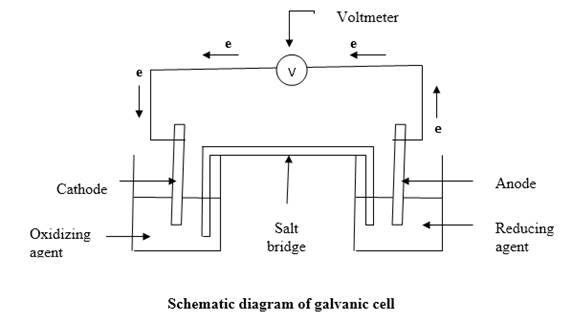
When reducing agent and oxidizing agent mixed together in a container, electron transfer occurs. But chemical energy generated by this cannot be used for any work. This can be achieved by separating the reducing agent and oxidizing agent and let the electron transfer happen through a wire. So electrons removing from the reducing agent will travel through the wire to the oxidizing agent. The electric current generated by the electron flow through the wire can be directed through an electronic device. To keep the net charge of each container zero, the two solutions are connected by a salt bridge which filled with strong electrolyte.
In the figure
According to the figure
Want to see more full solutions like this?
Chapter 18 Solutions
Introductory Chemistry: A Foundation
- Give the notation for a voltaic cell whose overall cell reaction is Mg(s)+2Ag+(aq)Mg2+(aq)+2Ag(s) What are the half-cell reactions? Label them as anode or cathode reactions. What is the standard cell potential of this cell?arrow_forwardElectrochemical Cells II Consider this cell running under standard conditions: Ni(s)Ni2(aq)Cu+(aq)Cu(s) a Is this cell a voltaic or an electrolytic cell? How do you know? b Does current flow in this cell spontaneously? c What is the maximum cell potential for this cell? d Say the cell is connected to a voltmeter. Describe what you might see for an initial voltage and what voltage changes, if any, you would observe as time went by. e What is the free energy of this cell when it is first constructed? f Does the free energy of the cell change over time as the cell runs? If so, how does it change?arrow_forward. In which direction do electrons flow in a galvanic cell, from anode to cathode or vice versa?arrow_forward
- The following two half-reactions arc involved in a voltaic cell. At standard conditions, what species is produced at each electrode? Ag++eAgE=0.80VNi2++2eNiE=0.25Varrow_forwardAs an example of an electrolytic cell, the text states: Sodium chloride is electrolyzed commercially in an apparatus called the Downs cell to produce sodium and chlorine. This is a high-temperature operation; the electrolyte is molten NaCl. Write the half-reaction equations for the changes taking place at each electrode. Is the electrode at which sodium is produced the anode or the cathode? The Downs cell electrolyzes molten melted sodium chloride, producing sodium and chlorine.arrow_forwardA mercury battery, used for hearing aids and electric watches, delivers a constant voltage (1.35 V) for long periods. The half-reactions are HgO(s)+H2O(l)+2eHg(l)+2OH(aq)Zn(s)+2OH(aq)Zn(OH)2(s)+2e Which half-reaction occurs at the anode and which occurs at the cathode? What is the overall cell reaction?arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





