
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 18, Problem 32P
Interpretation Introduction
Interpretation:
Compound that can remove
Concept introduction:
- Catalyst: Substance which helps in increasing the rate of a particular reaction without getting consumed in the reaction.
- Base Catalyst: A catalyst which helps in increasing the rate of a particular reaction by the removal of a proton. There are two types of catalysis:
- 1. Specific-base catalysis: Proton is completely removed before the slow step in a reaction
- 2. General- base catalysis: Proton is completely removed during the slow step in a reaction
- Intramolecular general-base catalysis: A catalysis where catalyst is a part of the molecule that undergoes the reaction promotes the reaction in a species by completely removing a proton during the slow step in a reaction
Expert Solution & Answer
Explanation of Solution
In the below compound, negatively charged oxygen atom is in axial position where it can easily undergo intramolecular general-base reaction comparing to
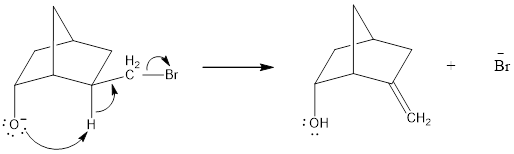
Therefore, the compound where
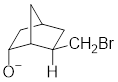
Want to see more full solutions like this?
Subscribe now to access step-by-step solutions to millions of textbook problems written by subject matter experts!
Students have asked these similar questions
The compound below has multiple electrophilic sites which could potentially react with an organolithium reagent, like phenyllithium. If only 1 mole of Phli is added for each mole of starting material, which atom is most likely to be attacked by the nucleophile?
Need your help for this:
Show the reaction mechanism for the formation of
a. RCONH2 from the reaction of RCOOR' with NH3
b. RCOOR" from the reaction of RCOOR' with R"OH in acidic medium
The reaction sequence shown below involves a diazonium ion intermediate. (image 1)
Draw a detailed mechanism that explains the formation of this diazonium ion. You do not need to show the formation of nitronium ion (NO+).
Chapter 18 Solutions
Essential Organic Chemistry, Global Edition
Ch. 18.1 - Prob. 1PCh. 18.2 - If H218O were used to hydrolyze lysozyme, which...Ch. 18.3 - Which of the following amino acid side chains can...Ch. 18.3 - Arginine and lysine side chains fit into trypsins...Ch. 18.4 - Which of the following amino acid side chains can...Ch. 18.4 - Prob. 6PCh. 18.5 - Prob. 7PCh. 18.5 - Draw the mechanism for the hydroxide-ion-catalyzed...Ch. 18.5 - What advantage does the enzyme gain by forming an...Ch. 18.7 - Prob. 10P
Ch. 18.7 - Prob. 11PCh. 18.8 - How many conjugated double bonds are there in a....Ch. 18.8 - Instead of adding to the 4a-position and...Ch. 18.8 - In succinate dehydrogenase, FAD is covalently...Ch. 18.8 - Prob. 15PCh. 18.9 - Acetolactate synthase is another TPP-requiring...Ch. 18.9 - Acetolactate synthase can also transfer the acyl...Ch. 18.9 - Prob. 18PCh. 18.9 - Prob. 19PCh. 18.10 - Prob. 21PCh. 18.11 - Prob. 23PCh. 18.11 - Which compound is more easily decarboxylated?Ch. 18.11 - Explain why the ability of PLP to catalyze an...Ch. 18.11 - Explain why the ability of PLP to catalyze an...Ch. 18.12 - What groups are interchanged in the following...Ch. 18.13 - Why is the coenzyme called tetrahydrofolate?Ch. 18.13 - What amino acid is formed by the following...Ch. 18.13 - How do the structures of tetrahydrofolate and...Ch. 18.13 - What is the source of the methyl group in...Ch. 18 - Prob. 32PCh. 18 - Prob. 33PCh. 18 - From what vitamins are the following coenzymes...Ch. 18 - Prob. 35PCh. 18 - For each of the following reaction, name both the...Ch. 18 - Explain why serine proteases do not catalyze...Ch. 18 - Prob. 38PCh. 18 - For each of the following enzyme catalyzed...Ch. 18 - Trisephosphate isomerase (TIM) catalyzes the...Ch. 18 - Prob. 41PCh. 18 - What acyl groups have we seen transferred by...Ch. 18 - When UMP is dissolved in T2O, exchange of T for H...Ch. 18 - Prob. 44PCh. 18 - When transaminated, the three branched-chain amino...Ch. 18 - Aldolase shows no activity if it is incubated with...
Knowledge Booster
Similar questions
- which will proceed more easily at room temperature the bromination of cyclohexene or the bromination of benzene?arrow_forwardA greener alternative to bromination with elemental bromine is the reaction of the acetanilide with potassium bromide and ammonium ceric nitrate. How does this reaction work?arrow_forwardWhat type of eliminaton reactions occur in this process?arrow_forward
- Who would compound X be in the following sequence of reactions? Select one:arrow_forwardWhich of the hydrogens will be abstracted first when mono-brominating with Br2 and light?arrow_forwardfor which of the following is NaOH a suitable reagent to deprotonate the indicated hydrogen quantitativelyarrow_forward
- In the mechanism involving the dehydration of an alcohol, which alkoxonium ion has the highest amount of energy? A. secondary alkoxonium ion B. methyloxonium ion C. tertiary alkoxonium ion D. primary alkoxonium ionarrow_forwardGiven that the Rho 0.61 explain which step presented is the rate determining step and why? Also explain the use of allyl stannate in the reaction.arrow_forwardWhich of these compounds are possible products in this reaction? (Choose all that apply.) A B C Darrow_forward
- We saw that acid anhydrides react with alcohols, water, and amines. In which of these reactions can the tetrahedral intermediate eliminate the carboxylate ion even if it does not lose a proton before the elimination step? Explainarrow_forwardWhich of the following compounds reacts most slowly during nitration?arrow_forwardWhich species is NOT part of the mechanism of the Fischer esterification reaction shown below?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT
