
(a)
Interpretation:
The main group elements on the periodic table has to be located.
Concept introduction:
Main group elements
Main group elements are
(a)
Explanation of Solution
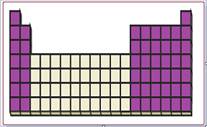
Figure 1
The location of s and p-block elements in the periodic table are shown using violet colored boxes in the above given figure 1.
(b)
Interpretation:
The
Concept introduction:
The s-block elements are (group 1) alkali metals and (group 2) alkaline earth metals.
(b)
Explanation of Solution
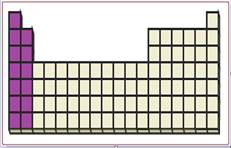
Figure 2
The s-block elements are (group 1) alkali metals and (group 2) alkaline earth metals. S-block elements have valence electrons in the
The location of s-block elements in the periodic table are shown using violet colored boxes in the above given figure 2.
(c)
Interpretation:
The
Concept introduction:
The p-block elements are groups
(c)
Explanation of Solution
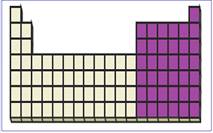
Figure 3
P-block elements have valence electrons in the
The location of p-block elements in the periodic table are shown using violet colored boxes in the above given figure 3.
(d)
Interpretation:
The main group metal elements on the periodic table has to be located.
Concept introduction:
Main group metals all are solid except mercury which is a liquid
Most have a silvery shine, good conductors of heat and electricity.
Relatively low ionization energies.
Relatively low electronegative.
(d)
Explanation of Solution
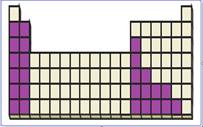
Figure 4
The location of main group metals in the periodic table are shown using violet colored boxes in the above given figure 1.
(e)
Interpretation:
The non-metal elements on the periodic table has to be located.
Concept introduction:
Non-metals all are gases at
Most lack a metallic luster
Relatively high ionization energies.
Relatively high electronegative.
Gain electrons to form anions, share electrons to form oxyanions
(e)
Explanation of Solution
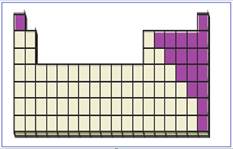
Figure 5
The location of main group non-metals in the periodic table are shown using violet colored boxes in the above given figure 5.
(f)
Interpretation:
The semimetals elements on the periodic table has to be located.
Concept introduction:
Carbon is a non-metal, silicon and germanium are semimetals, and tin and lead are metals. The horizontal and vertical periodic trends combine to locate the element with the most metallic character in the lower left of the periodic table, the element with the most non-metallic character in the upper right, and the semimetals along the diagonal stair step that stretches across the middle.
(f)
Explanation of Solution
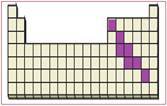
Figure 6
The location of semimetals in the periodic table are shown using violet colored boxes in the above given figure 6.
Want to see more full solutions like this?
Chapter 19 Solutions
General Chemistry: Atoms First
- (a) Mention the optimum conditions for the industrial manufacture of ammonia by Haber’s process. (b) Explain the following giving appropriate reasons: (i) Sulphur vapour exhibits paramagnetic behaviour: (ii) Red phosphorus is less reactive than white phosphorusarrow_forwardThe prefix eka- comes from the Sanskrit word for “one.”Mendeleev used this prefix to indicate that the unknownelement was one place away from the known element thatfollowed the prefix. For example, eka-silicon, which we nowcall germanium, is one element below silicon. Mendeleevalso predicted the existence of eka-manganese, which was notexperimentally confirmed until 1937 because this element isradioactive and does not occur in nature. Based on the periodictable shown in Figure 7.1, what do we now call the elementMendeleev called eka-manganese?arrow_forwardState reasons for the following observations: (i) The enthalpies of atomisation of transition elements are quite high. (ii) There is a greater horizontal similarity in the properties of the transition elements than of the main group elements.arrow_forward
- (a) What are the different oxidation states exhibited by the lanthanoids? (b) Write two characteristics of the transition elements. (c) Which of the 3d-block elements may not be regarded as the transition elements and why?arrow_forwardWhat happens when transition metals are oxidized?arrow_forwardWhy are fluorides of transition metals more stable in their higher oxidation state as compared to the lower oxidation state?arrow_forward
- Which chromium species will disproportionate in acid and base?arrow_forwardone of the following is true about the sodium metabisulfite a. oxidized to sulfonic acid b. it is an oxidizing agent c. gives 2 electron when oxidized by iodine d. it possess the highest possible oxidation number of sulfur atomarrow_forwardWhy is beryllium carbonate unusually unstable thermally as compared to the other carbonates of this group?arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning


