
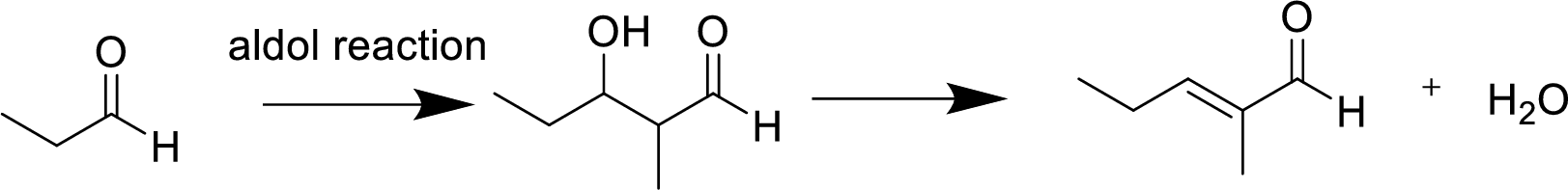
(a)
Interpretation:
The product of the aldol reaction of the given compound and the
Concept introduction:
Aldol reaction is an addition reaction of
(a)
Explanation of Solution
The aldol reaction product for the given compound has to be drawn.
The aldol reaction yield a
Base abstracts a proton from the
(b)
Interpretation:
The product of the aldol reaction of the given compound and the
Concept introduction:
Aldol reaction is an addition reaction of aldehydes and ketones. Aldol reaction is a reversible reaction and occurs in the presence of a strong base like sodium hydroxide. One molecule (aldehyde or ketone) acts a nucleophile and attacks the electrophilic carbon center of the other molecule to give the addition product. The product is named
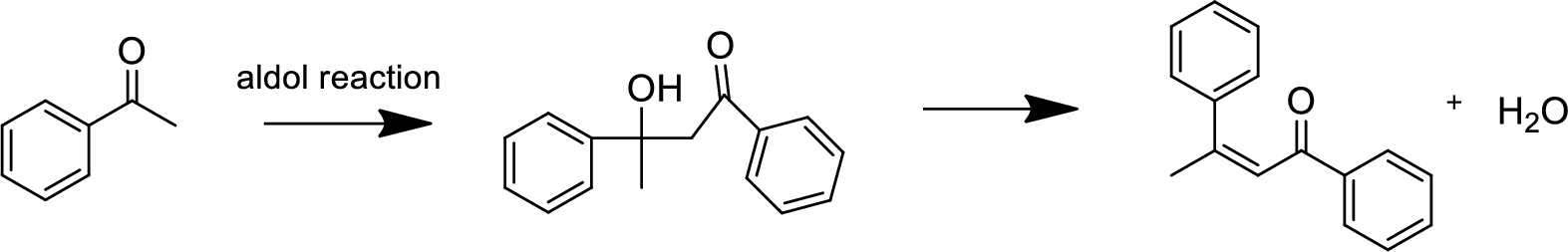
(b)
Explanation of Solution
The aldol reaction product for the given compound has to be drawn.
The aldol reaction yield a
Base abstracts a proton from the
(c)
Interpretation:
The product of the aldol reaction of the given compound and the
Concept introduction:
Aldol reaction is an addition reaction of aldehydes and ketones. Aldol reaction is a reversible reaction and occurs in the presence of a strong base like sodium hydroxide. One molecule (aldehyde or ketone) acts a nucleophile and attacks the electrophilic carbon center of the other molecule to give the addition product. The product is named
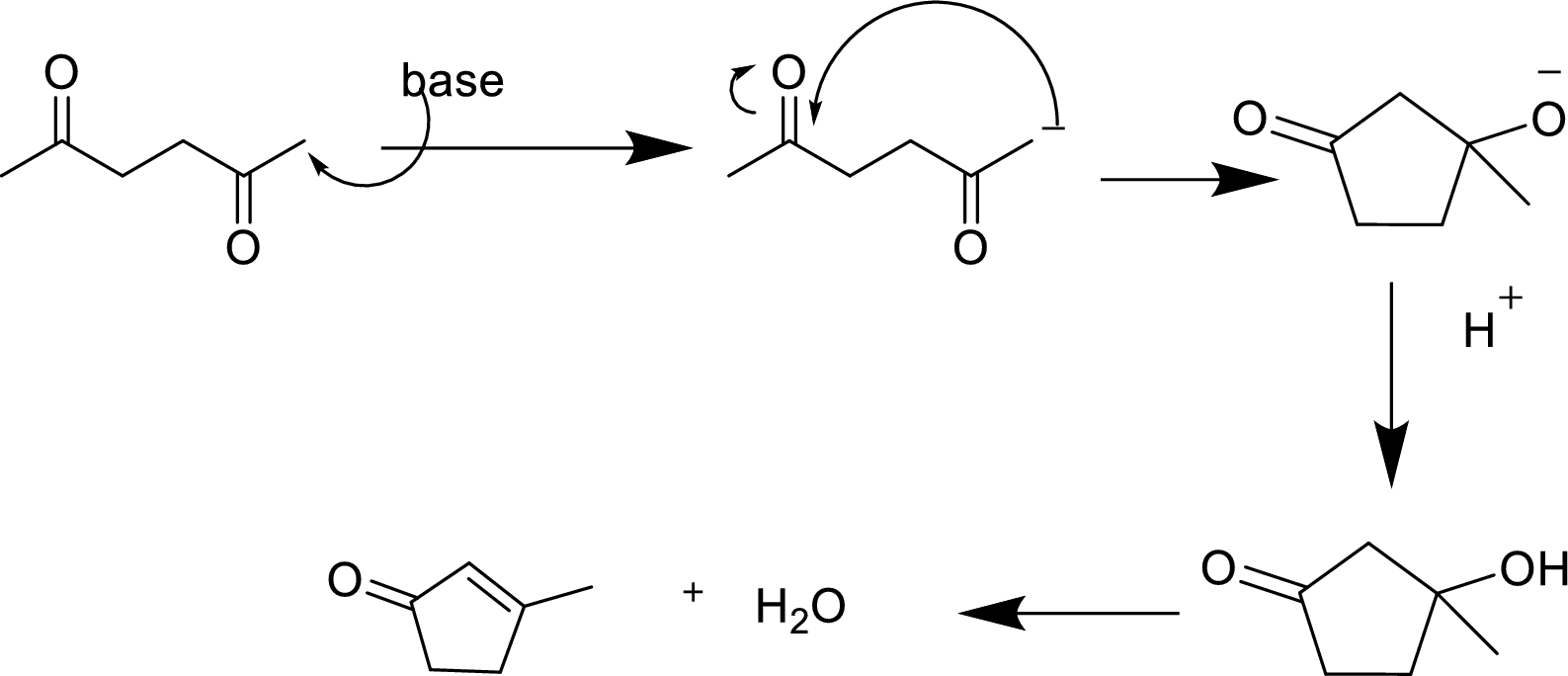
(c)
Explanation of Solution
The aldol reaction product for the given compound has to be drawn.
The aldol reaction yield a
Base abstracts a proton from the
Want to see more full solutions like this?
Chapter 19 Solutions
Organic Chemistry
- Give the products from the following aldol reactionarrow_forwardGive the structures of the aldol products that form when each of the following compounds or mixtures is treated with NaOH. The top three compounds are aldol dimerizations and require 2 moles of the reactant listed, while the two compounds on the bottom row are being used in a mixed aldol reaction. Butanal, Cyclopentanone, and Acetophenone (PhCOCH3) p-chlorobenzaldehyde and 2,2-dimethylcyclohexanonearrow_forwardWhat is the product resulted due to the reaction of 2-hydroxy-3-methoxybenzaldehyde with ethyl bromoacetate in a basic solution indicating all the reagents and intermediates occurred during the reaction? What is the product obtained when product A reacted with ethyl acetate in the same basic solution?arrow_forward
- The synthesis of carbohydrates can be particularly difficult because of the large number of chiral centers and OH functional groups present. Epoxides can be useful synthetic intermediates in carbohydrate syntheses. Draw the product of the following reaction of a Gilman reagent with each epoxidearrow_forwardDraw the product of the base-catalyzed aldol reaction of compound. Q.) Cyclopentanonearrow_forwardWhat is the first step in a base catalyzed aldol reaction? A. Protonation of Carbonyl group B. Elimination of Carbonyl with an alkene C. Formation of an Enolate D. Elimination of hydroxide E. Addition of nucleophile to R2C=Oarrow_forward
- Provide a detailed mechanism for the Aldol-Michael reaction sequence using acetophenone and pyridine- 2-carboxaldehyde to make 1,5-diphenyl-3-(pyridin-2-yl)pentane-1,5-dionearrow_forwardUsing cyclopentanone as the reactant, show the product of a. acid-catalyzed keto–enol interconversion. b. an aldol addition. c. an aldol condensation.arrow_forwardPredict the products of aldol condensation, followed by dehydration, of the followingketones and aldehydes.(a) butyraldehyde (b) acetophenonearrow_forward
- A compound known as Hagemann’s ester can be prepared by treating a mixture of formaldehyde and ethyl acetoacetate first with base and then with acid and heat. Write the structure for the product of each of the steps.a. The first step is an aldol-like condensation.b. The second step is a Michael addition.c. The third step is an intramolecular aldol addition.d. The final transformation includes a dehydration and a hydrolysis followed by a decarboxylation.arrow_forwardWhat is the structural requirement of the following reactions? 1) Iodoform Reaction 2) Aldol Condensationarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


