
Concept explainers
Using the
a. 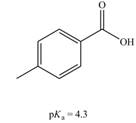 b.
b. 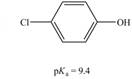 c.
c. 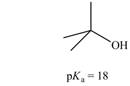
(a)
Interpretation: The correct
Concept introduction: Carboxylic acids are the carbon compounds that contain carboxyl group as a major functional group. They are polar due to electronegativity difference between the atoms in a compound. They sometimes exist as a dimer. Dimers are the compounds that consist of two monomer units connected by bonds or forces. Carboxylic acids are synthesized from alkynes, alkene, benzene derivatives, alcohol and allylic halides by using different reagents.
Answer to Problem 19.35P
The bases that are strong enough to deprotonate the given compounds are
Explanation of Solution
The given carboxylic acid is
The
Therefore, the bases that are strong enough to deprotonate the given compounds are
The bases that are strong enough to deprotonate the given compounds are
(b)
Interpretation: The correct
Concept introduction: Carboxylic acids are the carbon compounds that contain carboxyl group as a major functional group. They are polar due to electronegativity difference between the atoms in a compound. They sometimes exist as a dimer. Dimers are the compounds that consist of two monomer units connected by bonds or forces. Carboxylic acids are synthesized from alkynes, alkene, benzene derivatives, alcohol and allylic halides by using different reagents.
Answer to Problem 19.35P
The bases that are strong enough to deprotonate the given compounds are
Explanation of Solution
The given compound is
The
Therefore, the bases that are strong enough to deprotonate the given compounds are
The bases that are strong enough to deprotonate the given compounds are
(c)
Interpretation: The correct
Concept introduction: Carboxylic acids are the carbon compounds that contain carboxyl group as a major functional group. They are polar due to electronegativity difference between the atoms in a compound. They sometimes exist as a dimer. Dimers are the compounds that consist of two monomer units connected by bonds or forces. Carboxylic acids are synthesized from alkynes, alkene, benzene derivatives, alcohol and allylic halides by using different reagents.
Answer to Problem 19.35P
The bases that are strong enough to deprotonate the given compounds are
Explanation of Solution
The given carboxylic acid is
The
Therefore, the bases that are strong enough to deprotonate the given compounds are
The bases that are strong enough to deprotonate the given compounds are
Want to see more full solutions like this?
Chapter 19 Solutions
Organic Chemistry
- 16-26 The p/fb of amphetamine is approximately 3.2 Amphetamine (a) Which form of amphetamine (the free base or its conjugate acid) would you expect to be present at pH 1.0, the pH of stomach acid? (b ) Which form of amphetamine would you expect to be present at pH 7.40, the pH of blood plasma?arrow_forwardRank the following substances in order of increasing acidity: (a) (CH3)2CHOH, HC≡CH, (CF3)2CHOH, CH3OH (b) Phenol, p-methylphenol, p-[trifluoromethyl) phenol (c) Benzyl alcohol, phenol, p-hydroxybenzonic acidarrow_forwardWill acetylene react with sodium hydride according to the following equation to form a salt and hydrogen, H2? Using pKa values given in Table 4.1, calculate Keq for this equilibrium.arrow_forward
- In each pair, select the stronger acid. (a) Pyruvic acid (pKa 2.49) or lactic acid (pKa 3.08) (b) Citric acid (pKa1 3.08) or phosphoric acid (pKa1 2.10)arrow_forwardCalculate the Ka's for the following acids: (a) Citric acid, pKa = 3.14 (b) Tartaric acid, pKa = 2.98arrow_forwardUsing pKa Values to Determine Relative Acidity and Basicity Rank the following compounds in order of increasing acidity, and then rank their conjugate bases in order of increasing basicity.arrow_forward
- a. Rank the following carboxylic acids from strongest to weakest acid: CH3CH2CH2COOH CH3CH2CHCOOH ClCH2CH2CH2COOH CH3CHCH2COOH b. How does the presence of an electronegative substituent such as Cl affect the acidity of a carboxylic acid? c. How does the location of the substituent affect the acidity of the carboxylic acid?arrow_forwardAn unknown compound, X, is thought to have a carboxyl group with a p?a of 2.5 and a second ionizable group with a p?a between 5.0 and 8.0. When 50.0 mL of 0.2 M NaOH is added to 75.0 mL of a 0.2 M solution of X at pH 2.5, the pH increases to 7.13. Calculate the p?a of the second ionizable group of X.arrow_forwardDetermine which of the following bases is strong enough to deprotonate acetonitrile (CH3CN), so that equilibrium favors the products:(a) NaH; (b) Na2CO3; (c) NaOH; (d) NaNH2; (e) NaHCO3.arrow_forward
- What is the K a of an acid whose pK a is 8.60?arrow_forwardWhich of the ff statements is false? In aqueous solution the order of base strength R3N > R2NH > RNH2 > NH3. Alkyl groups increase the electron density on nitrogen, making the amine more basic. Alkyl groups stabilize the positive charge on the conjugate acid making the ammonium ion less acidic. Solvation through H-bonding with water is more important in the conjugate acid than in the free amine.arrow_forwardWhat is the conjugate acid of H2PO4-1? Select one: a. H3PO4 b. HPO4-2 c. PO4-3 d. H4PO4+1arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


