
Concept explainers
Identify each compound from its spectral data.
(a)
Interpretation: The given compound is to be identified from its spectral data.
Concept introduction: IR spectroscopy is used to identify the functional group present in a compound. Each and every bond vibrates at a characteristic frequency. Proton
Answer to Problem 19.57P
The given compound from its spectrum data of IR, and
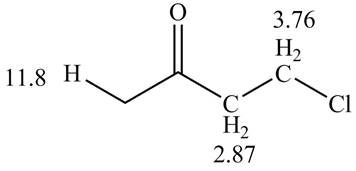
Figure 1
Explanation of Solution
The spectral data for the given compound is as follows:
IR absorptions at
Information from
The observed chemical shift value at
The observed chemical shift value at
The observed chemical shift values at
Thus, the structure of a given compound from its spectrum data of IR, and
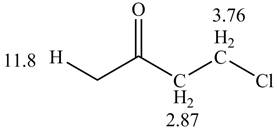
Figure 1
The given compound from its spectrum data of IR, and
(b)
Interpretation: The given compound is to be identified from its spectral data.
Concept introduction: IR spectroscopy is used to identify the functional group present in a compound. Each and every bond vibrates at a characteristic frequency. Proton
Answer to Problem 19.57P
The structure of a given compound from its spectrum data of IR, and
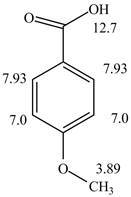
Figure 2
Explanation of Solution
The spectral data for the given compound is as follows:
IR absorptions at
Information from
The observed chemical shift values at
The observed chemical shift value at
The observed chemical shift value at
Thus, the structure of a given compound from its spectrum data of IR, and
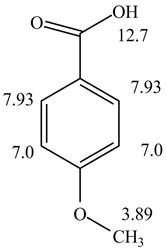
Figure 2
The given compound from its spectrum data of IR, and
(c)
Interpretation: The given compound is to be identified from its spectral data.
Concept introduction: IR spectroscopy is used to identify the functional group present in a compound. Each and every bond vibrates at a characteristic frequency. Proton
Answer to Problem 19.57P
The given compound from its spectrum data of IR, and
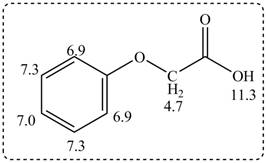
Figure 3
Explanation of Solution
The spectral data for the given compound is as follows:
IR absorptions at
Information from
The observed chemical shift values at
The observed chemical shift value at
The observed chemical shift value at
Thus, the structure of a given compound from its spectrum data of IR, and
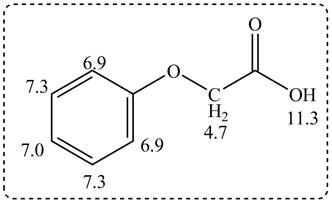
Figure 3
The given compound from its spectrum data of IR, and
Want to see more full solutions like this?
Chapter 19 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Organic Chemistry
CHEMISTRY-TEXT
Chemistry: The Central Science (13th Edition)
Chemistry & Chemical Reactivity
Organic Chemistry - Standalone book
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
- Following are 1H-NMR spectra for compounds G, H, and I, each with the molecular formula C5H12O. Each is a liquid at room temperature, is slightly soluble in water, and reacts with sodium metal with the evolution of a gas. (a) Propose structural formulas of compounds G, H, and I. (b) Explain why there are four lines between 0.86 and 0.90 for compound G. (c) Explain why the 2H multiplets at 1.5 and 3.5 for compound H are so complex.arrow_forwardPropose a structure consistent with the following spectral data for a compound C8H18O2: IR: 3350 cm-1 1H NMR: 1.24 δ (12 H, singlet); 1.56 δ (4 H, singlet); 1.95 δ (2 H, singlet)arrow_forwardAssume a compound with the formula C4H8O. a) How many double bonds and/or rings does your compound contain? b) If your compound shows an infrared absorption peak at 1715 cm-1, what functional group does it have? c) If your compound shows a single 1H NMR absorption peak at 2.1 δ, what is its structure?arrow_forward
- Propose a structure consistent with each set of data.a. a compound that contains a benzene ring and has a molecular ion at m/z = 107b. a hydrocarbon that contains only sp3 hybridized carbons and a molecular ion at m/z = 84c. a compound that contains a carbonyl group and gives a molecular ion at m/z = 114d. a compound that contains C, H, N, and O and has an exact mass for the molecular ion at 101.0841arrow_forwardPropose a structure consistent with each set of spectral data: a.C6H14O: IR peak at 3600−3200 cm−1; NMR (ppm): 0.8 (triplet, 6 H) 1.5 (quartet, 4 H) 1.0 (singlet, 3 H) 1.6 (singlet, 1 H) b.C6H14O: IR peak at 3000−2850 cm−1; NMR (ppm): 1.10 (doublet, relative area = 6) 3.60 (septet, relative area = 1)arrow_forwardIdentify below compound from its spectral data. Molecular formula:C8H8O3 IR:3500–2500 cm−1, 1710 cm−1 1H NMR data:4.7 (singlet, 2 H), 6.9–7.3 (multiplet, 5 H), and 11.3(singlet, 1 H) ppmarrow_forward
- 15. Compound C has a molecular weight of 94.54 and the 1H-NMR spectrum shows four signals - a triplet at 3.81 ppm, a triplet at 3.63 ppm, a singlet at 2.19 ppm, and a triplet of triplets at 2.02 ppm. The mass, IR, and 13C-NMR spectra of compound C are shown below, they are also downloadable for closer inspection by clicking the link under the spectral data. Identify C and explain your reasoning. refer to picturearrow_forwardPropose a structure consistent with each set of data. a.a compound that contains a benzene ring and has a molecular ion at m/z = 107 b.a hydrocarbon that contains only sp3 hybridized carbons and a molecular ion at m/z = 84 c.a compound that contains a carbonyl group and gives a molecular ion at m/z = 114 d.a compound that contains C, H, N, and O and has an exact mass for the molecular ion at 101.0841arrow_forwardA compound used as a moth repellant has three molecular ion peaks at m/z = 146 (100%), 148 (65%) & 150 (10%) amu in its mass spectrum. A pair of smaller peaks are seen at m/z= 111 (34%) & 113 (11%). The infrared spectrum shows sharp absorption just above 3000 cm^-1 region, and also at 1480 cm^-1. The ^1 H nmr shows a single sharp signal at delta = 7.2 ppm, and the ^13 C nmr has two signals (delta = 133 & 130 ppm).arrow_forward
- Compound B has molecular formula C9H12. It shows five signals in the 1H-NMR spectrum - a doublet of integral 6 at 1.22 ppm, a septet of integral 1 at 2.86 ppm, a singlet of integral 1 at 5.34 ppm, a doublet of integral 2 at 6.70 ppm, and a doublet of integral 2 at 7.03 ppm. The 13C-NMR spectrum of B shows six unique signals (23.9, 34.0, 115.7, 128.7, 148.9, and 157.4). Identify B and explain your reasoning.arrow_forwardAn unknown compound C3H2NCl shows moderately strong IR absorptions around 1650 cm-1 and 2200 cm-1. Its NMR spectrum consists of two doublets (J = 14 Hz) at δ 5.9 and δ 7.1. Propose a structure consistent with this data?arrow_forwardPropose a structure consistent with following set of spectral data: C5H10O2: IR peak at 1740 cm−1; NMR (ppm): 1.15 (triplet, 3 H) 2.30 (quartet, 2 H) 1.25 (triplet, 3 H) 4.72 (quartet, 2 H)arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

