
Concept explainers
(a)
Interpretation:
The percentage of atoms having velocity within
Concept introduction:
The probability distribution function of the velocities of the gas molecules in each dimension is given by
The Maxwell-Boltzmann distribution is shown below.
This distribution depends on the mass of the particle and absolute temperature.
Answer to Problem 19.73E
The percentage of atoms having a velocity within
Explanation of Solution
The given temperature is
The molar mass of the helium gas is
The graph of
Figure 1
The formula to calculate the root-mean-square speed is given below.
Where,
•
•
•
Substitute the values the value of the molar mass of helium,
The corresponding value of the percentage of atoms having
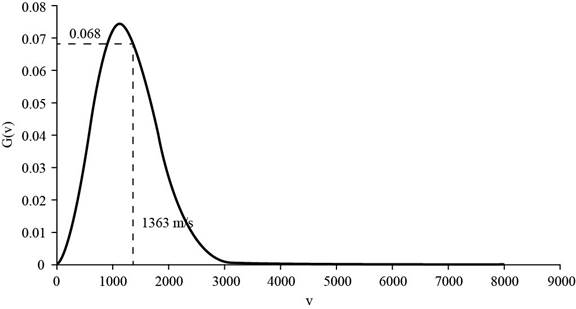
Figure 2
Therefore, the percentage of atoms having velocity within
The percentage of atoms having velocity within
(b)
Interpretation:
The percentage of atoms having velocity within
Concept introduction:
The probability distribution function of the velocities of the gas molecules in each dimension is given by
The Maxwell-Boltzmann distribution is given by,
This distribution depends on the mass of the particle and absolute temperature.
Answer to Problem 19.73E
The percentage of atoms having velocity within
Explanation of Solution
The given temperature is
The molar mass of the helium gas is
The graph of
Figure 1
The formula to calculate the most probable speed is given below as,
Where,
•
•
•
Substitute the values the value of the molar mass of helium,
The corresponding value of the percentage of atoms having
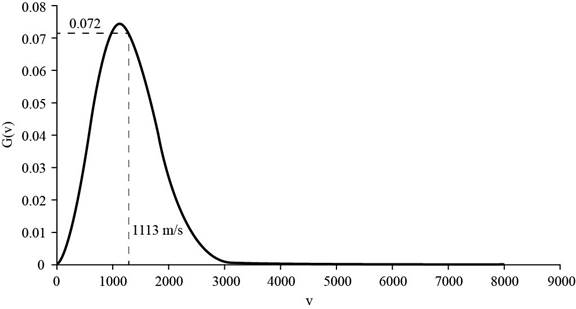
Figure 3
Therefore, the percentage of atoms having velocity within
The percentage of atoms having velocity within
(c)
Interpretation:
The percentage of atoms having velocity within
Concept introduction:
The probability distribution function of the velocities of the gas molecules in each dimension is given by
The Maxwell-Boltzmann distribution is given by,
This distribution depends on the mass of the particle and absolute temperature.
Answer to Problem 19.73E
The percentage of atoms having velocity within
Explanation of Solution
The given temperature is
The molar mass of the helium gas is
The graph of
Figure 1
The formula to calculate the mean speed is given below as,
Where,
•
•
•
Substitute the values the value of molar mass of helium,
The corresponding value of percentage of atoms having
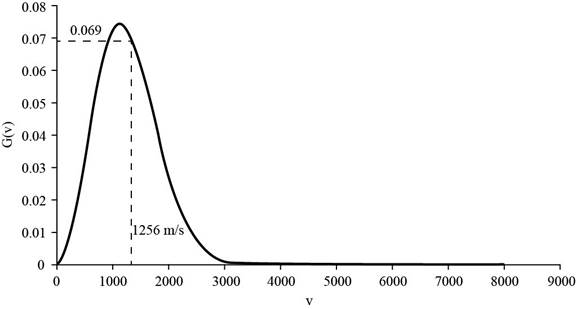
Figure 4
Therefore, the percentage of atoms having velocity within
The percentage of atoms having velocity within
The percentage of atoms having velocity within
Therefore, all the percentages have a relative same value.
The percentage of atoms having velocity within
Want to see more full solutions like this?
Chapter 19 Solutions
Physical Chemistry
- Oxygen Consumption If 5.00 L of hydrogen gas,measured at a temperature of 20.0°C and a pressure of80.1 kPa, is burned in excess oxygen to form water, whatmass of oxygen will be consumed? Assume temperatureand pressure remain constant.arrow_forwardGiven that a sample of air is made up of nitrogen, oxygen, and argon in the mole fractions 0.78 N2, 0.21 O2, and 0.010 Ar, what is the density of air at standard temperature and pressure?arrow_forwardWhat is the ratio of the average kinetic energy of a SO2 molecule to that of an O2 molecule in a mixture of two gases? What is the ratio of the root mean square speeds, urms, of the two gases?arrow_forward
- SF6 is a gas at room temperature, 295K. What is its root-mean-square speed at that temperature?arrow_forwardOne of the chemical controversies of the nineteenth century concerned the element beryllium (Be). Berzelius originally claimed that beryllium was a trivalent element (forming Be3+ ions) and that it gave an oxide with the formula Be2O3. This resulted in a calculated atomic mass of 13.5 for beryllium. In formulating his periodic table, Mendeleev proposed that beryllium was divalent (forming Be2+ ions) and that it gave an oxide with the formula Be2O3. This assumption gives an atomic mass of 9.0. In 1894, A. Combes (Comptes Rendus 1894, p. 1221) reacted beryllium with the anion C5H7O2and measured the density of the gaseous product. Combess data for two different experiments are as follows: I II Mass 0.2022 g 0.2224 g Volume 22.6 cm3 26.0 cm3 Temperature 13C 17C Pressure 765.2 mm Hg 764.6 mm If beryllium is a divalent metal, the molecular formula of the product will be Be(C5H7O2)2; if it is trivalent, the formula will be Be(C5H7O2)3. Show how Combess data help to confirm that beryllium is a divalent metal.arrow_forwardIf 456 dm3 of krypton at 101 kPa and 21C is compressed into a 30.1-dm3 tank at the same temperature, what is the pressure of krypton in the tank?arrow_forward
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





