
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 19, Problem 19.8P
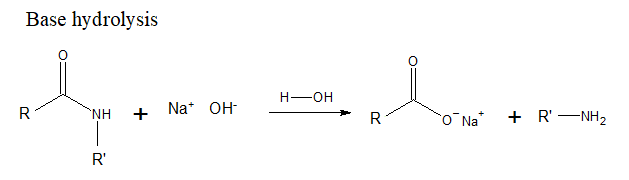
(a)
Interpretation Introduction
Interpretation: The products formed when benzamide, C6H5CONH2is treated with the following set of reagents should be determined.
H2O, NaOH, heat
Concept Introduction: Basic hydrolysis with NaOH causes an acid amide to form sodium salt of the
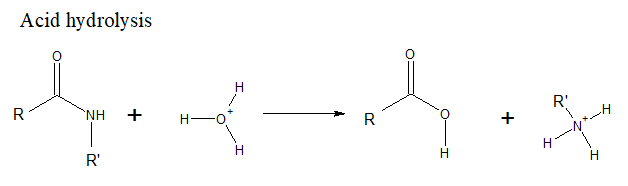
(b)
Interpretation Introduction
Interpretation: The products formed when benzamide, C6H5CONH2is treated with the following set of reagents should be determined.
H2O, HCl, heat
Concept Introduction: Acid hydrolysis with HCl causes an acid amide to form carboxylic acid and amine.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
If a carboxylic acid is dissolved in isotopically labeled methanol (CH3
18OH) and an acid catalyst is added, where will the label reside in the product?
Complete each of the following by supplying the missing reagents. Draw the structures of each of the reactants and products.
a. N-Methylpropanamide + ? --> propanoic acid + ?
b. N,N-Dimethylacetamide + strong acid --> ? + ?
c. Formamide + strong acid --> ? + ?
In a 0.28 mM aqueous solution of trimethylacetic acid (C4H9CO2H), what is the percentage of trimethylacetic acid that is dissociated?
Chapter 19 Solutions
Introduction to General, Organic and Biochemistry
Ch. 19.1 - Prob. 19.1PCh. 19.4 - Problem 19-2 Complete the equation for each...Ch. 19.4 - Prob. 19.3PCh. 19 - Prob. 19.4PCh. 19 - Write the IUPAC name for each compound.Ch. 19 - Prob. 19.6PCh. 19 - Prob. 19.7PCh. 19 - Prob. 19.8PCh. 19 - Prob. 19.9PCh. 19 - 0 Complete the equations for these reactions.
Ch. 19 - Prob. 19.11PCh. 19 - Prob. 19.12PCh. 19 - Prob. 19.13PCh. 19 - Prob. 19.14PCh. 19 - Prob. 19.15PCh. 19 - 6 Why are Dacron and Mylar referred to as...Ch. 19 - 7 What type of structural feature do the...Ch. 19 - Prob. 19.18PCh. 19 - Prob. 19.19PCh. 19 - 0 Show how triphosphoric acid can form from three...Ch. 19 - 1 Write an equation for the hydrolysis of...Ch. 19 - 2 (Chemical Connections 19A) Locate the ester...Ch. 19 - Prob. 19.23PCh. 19 - Prob. 19.24PCh. 19 - Prob. 19.25PCh. 19 - Prob. 19.26PCh. 19 - Prob. 19.27PCh. 19 - 8 (Chemical Connections 19C) Once it has been...Ch. 19 - Prob. 19.29PCh. 19 - Prob. 19.30PCh. 19 - Prob. 19.31PCh. 19 - Prob. 19.32PCh. 19 - Prob. 19.33PCh. 19 - 4 (Chemical Connections 19F) Why do Lactomer...Ch. 19 - Prob. 19.35PCh. 19 - Prob. 19.36PCh. 19 - Prob. 19.37PCh. 19 - 8 In Chapter 22, we will discuss a class of...Ch. 19 - Prob. 19.39PCh. 19 - Prob. 19.40PCh. 19 - Prob. 19.41PCh. 19 - Prob. 19.42PCh. 19 - Prob. 19.43PCh. 19 - Prob. 19.44PCh. 19 - Prob. 19.45PCh. 19 - Prob. 19.46PCh. 19 - Prob. 19.47PCh. 19 - Prob. 19.48PCh. 19 - Prob. 19.49P
Knowledge Booster
Similar questions
- If N,N-diethyl-1-pentanamine reacts with 3-Methylhexanoic acid, draw the resulting skeletal structure and provide its name?arrow_forwardWrite reaction of ethylmethylamine with the following reagents: a. HNO3 b. CH3COClarrow_forwardDraw and name compounds that meet the following descriptions a.Three acid chlorides having the formula C6H9ClO b. Three amides having the formula C7H11NOarrow_forward
- When cyclohexanone was reacted with an amine A. It the reaction formed an enamine, which of the following is the most likely identity of amine A? CH3N(CH3)2 CH3NH2 CH3NHCH3 NH3arrow_forwardDraw a structural formula for the product formed by treating butanal with reagent. Q.)HOCH2CH2OH, HClarrow_forwardEthyl butyrate, CH3CH2CH2CO2CH2CH3CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring. It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l) Part A Given 7.30 gg of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100%% yield? Express your answer in grams to three significant figures. Part B A chemist ran the reaction and obtained 5.95 gg of ethyl butyrate. What was the percent yield? Express your answer as a percent to three significant figures. Part C The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0%% yield. How many grams would be produced from 7.30 gg of…arrow_forward
- Ethyl butyrate, CH3CH2CH2CO2CH2CH3CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring. It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l) Given 8.45 gg of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100%% yield? Express your answer in grams to three significant figures. A chemist ran the reaction and obtained 5.50 gg of ethyl butyrate. What was the percent yield? Express your answer as a percent to three significant figures. The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0%% yield. How many grams would be produced from 8.45 gg of butanoic acid and excess…arrow_forwardEthyl butyrate, CH3CH2CH2CO2CH2CH3CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring. It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l)CH3CH2CH2CO2H(l)+CH2CH3OH(l)⟶H+CH3CH2CH2CO2CH2CH3(l)+H2O(l) A chemist ran the reaction and obtained 5.40 g of ethyl butyrate. What was the percent yield, The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0% yield. How many grams would be produced from 7.45g of butanoic acid and excess ethanol?arrow_forwardWhat happens when phenol is oxidized by Na2Cr2O7/H2SO4?arrow_forward
- Draw a structural formula of the principal product formed when ethyl benzoate is treated with reagent. Q.) C6H5MgBr (two equivalents), then HCl/H2Oarrow_forwardWrite the explicit structures of the following compounds. a) 2-methylbütannitril b) N-ethyl-N-methylmethanamite c) ethanoylpropanoate d) 3-methylanisol e) 3-ethylhexanedioic acidarrow_forward(CH3CO2CH2C6H5) is a naturally occurring ester in peaches. what hydrolysis products are formed when water is treated with water and NaOH?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning