
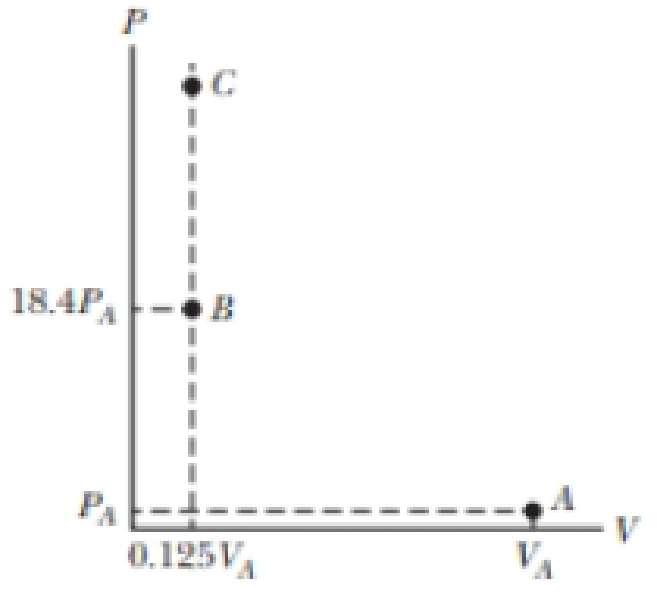
You have a particular interest in automobile engines, so you have secured a co-op position at an automobile company while you attend school. Your supervisor is helping you to learn about the operation of an internal combustion engine. She gives you the following assignment, related to a simulation of a new engine she is designing. A gas, beginning at PA = 1.00 atm, VA = 0.500 L, and TA = 27.0°C, is compressed from point A on the PV diagram in Figure P19.31 (page 530) to point B. This represents the compression stroke in a fourcycle gasoline engine. At that point, 132 J of energy is delivered to the gas at constant volume, taking the gas to point C. This represents the transformation of potential energy in the gasoline to internal energy when the spark plug fires. Your supervisor tells you that the internal energy of a gas is proportional to temperature (as we shall find in Chapter 20), the internal energy of the gas at point A is 200 J, and she wants to know what the temperature of the gas is at point C.
Figure P19.31
Want to see the full answer?
Check out a sample textbook solution
Chapter 19 Solutions
Physics for Scientists and Engineers with Modern Physics
- If a gas is compressed isothermally, which of the following statements is true? (a) Energy is transferred into the gas by heat. (b) No work is done on the gas. (c) The temperature of the gas increases. (d) The internal energy of the gas remains constant. (e) None of those statements is true.arrow_forwardOne mole of an ideal gas does 3 000 J of work on its surroundings as it expands isothermally to a final pressure of 1.00 atm and volume of 25.0 L. Determine (a) the initial volume and (b) the temperature of the gas.arrow_forwardA heat engine with 0.221 mol of a monatomic gas undergoes the cyclic procedure shown in the ?? diagram, where ?1=360 kPa, ?2=480 kPa, ?1=1.52×103 cm3, and ?2=2.34×103 cm3. Between Stage 3 and Stage 1, the gas is at a constant temperature, and between Stage 2 and Stage 3, no heat is transferred in or out. The temperature of the gas at Stage 2 is 400 K. Identify the type of each process in the cycle. The process between Stage 1 and Stage 2 is The process between Stage 2 and Stage 3 is The process between Stage 3 and Stage 1 is What is the temperature ? between Stages 3 and 1? ?= What is the work ?out per cycle of this heat engine? ?out= Find the efficiency ? of this heat engine. ?=arrow_forward
- A heat engine with 0.221 mol of a monatomic gas undergoes the cyclic procedure shown in the ?? diagram, where ?1=360 kPa, ?2=480 kPa, ?1=1.52×103 cm3, and ?2=2.34×103 cm3. Between Stage 3 and Stage 1, the gas is at a constant temperature, and between Stage 2 and Stage 3, no heat is transferred in or out. The temperature of the gas at Stage 2 is 400 K. Identify the type of each process in the cycle. The process between Stage 1 and Stage 2 is The process between Stage 2 and Stage 3 is The process between Stage 3 and Stage 1 is What is the temperature ? between Stages 3 and 1? ?= K What is the work ?out per cycle of this heat engine? ?out= J Find the efficiency ? of this heat engine. ?=arrow_forwardConsider a Carnot engine operating between 75 degrees Celsius and 25 degrees Celsius using one mole of an ideal, diatomic gas. If V1 = 0.01 cubic meter and V2 = 0.10 cubic meter, answer the questions that follow. (A). What is V3 (in m³)? Express answer in THREE SIGNIFICANT FIGURES? (B). What is V4 (in m³)? Express answer in THREE SIGNIFICANT FIGURES? (C). What is qh (in Joules)? Express answer in THREE SIGNIFICANT FIGURES? (D). What is qc (in Joules)? Express answer in THREE SIGNIFICANT FIGURES? (E). What is the total work(in Joules)? Express answer in THREE SIGNIFICANT FIGURES?arrow_forwardSketch a PV diagram and find the work done by the gas during the following stages. A. A gas is expanded from a volume of 1.0 L to 7.0 L at a constant pressure of 8.0 atm. __ J B. The gas is then cooled at constant volume until the pressure falls to 1.5 atm. C. The gas is then compressed at a constant pressure of 1.5 atm from a volume of 7.0 L to 1.0 L. D. The gas is heated until its pressure increases from 1.5 atm to 8.0 atm at a constant volume. E. Find the net work done during the complete cycle. F. Sketch a PV diagram of the process outlined in parts A - D.arrow_forward
- Sketch a PV diagram and find the work done by the gas during the following stages. Answer all in J. (a) A gas is expanded from a volume of 1.0 L to 8.0 L at a constant pressure of 4.5 atm. (b) The gas is then cooled at constant volume until the pressure falls to 1.5 atm. (c) The gas is then compressed at a constant pressure of 1.5 atm from a volume of 8.0 L to 1.0 L. (Note: Be careful of signs.) (d) The gas is heated until its pressure increases from 1.5 atm to 4.5 atm at a constant volume. (e) Find the net work done during the complete cycle. Sketch a PV diagram of the process outlined in parts (a)–(d).arrow_forwardAn ideal diatomic gas contracts in an isobaric process from 1.35 m3 to 0.600 m3 at a constant pressure of 1.70 ✕ 105 Pa. If the initial temperature is 455 K, find the work done on the gas, the change in internal energy, the energy transfer Q, and the final temperature. (a) the work done on the gas (in J) J (b) the change in internal energy (in J) J (c) the energy transfer Q (in J) J (d) the final temperature (in K) Karrow_forwardSketch a PV diagram and find the work done by the gas during the following stages. (a) A gas is expanded from a volume of 1.0 L to 3.0 L at a constant pressure of 3.0 atm. (b) The gas is then cooled at constant volume until the pressure falls to 2.0 atm. (c) The gas is then compressed at a constant pressure of 2.0 atm from a volume of 3.0 L to 1.0 L. Note: Be careful of signs. (d) The gas is heated until its pressure increases from 2.0 atm to 3.0 atm at a constant volume. (e) Find the net work done during the complete cycle.arrow_forward
- One mole of gas initially at a pressure of 2.00 atm and a volume of 0.300 L has an internal energy equal to 91.0 J. In its final state, the gas is at a pressure of 1.50 atm and a volume of 0.800 L, and its internal energy equals 182 J. For the paths IAF, IBF, and IF in Figure P12.30, calculate (a) the work done on the gas and (b) the net energy transferred to the gas by heat in the process.arrow_forwardA gas in a cylinder is held at a constant pressure of 2.20×105 Pa and is cooled and compressed from 1.90 m3 to 1.10 m3 . The internal energy of the gas decreases by 1.15×105 J. a) Find the work done by the gas. Express your answer in joules b)Find the amount of the heat that flowed into or out of the gas. Express your answer in joules to two significant figures. c) State the direction (inward or outward) of the flow.arrow_forwardThe initial pressure and volume of a diatomic gas (? = 1.40) immediately after combustion inside an automobile engine are 2.83 ✕ 106 Pa and 5.89 ✕ 10−5 m3 respectively. In the next step of the cycle, the piston moves outward allowing the gas to expand until its final volume is 2.85 ✕ 10−4 m3. Note that during this expansion, there is no energy transfer. Determine the work done during the expansion of the gas. ______Jarrow_forward
 Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning



