
Draw the structure for each of the following:
a. phenol
b. benzyl phenyl ether
c. benzonitrile
d. benzaldehyde
e. anisole
f. styrene
g. toluene
h. tert-buty lbenzene
i. benzyl chloride
a)
Interpretation:
The structure of phenol is to be drawn.
Concept introduction:
Phenols are defined as those compounds in which hydroxy group is attached directly to the benzene ring. Phenols and alcohols have so many similar properties.
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 46P
The structure of phenol is shown below.
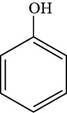
Figure 1
Explanation of Solution
The structure of phenol is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of phenol is shown below.
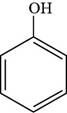
Figure 1
b)
Interpretation:
The structure of benzyl phenyl ether is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 46P
The structure of benzyl phenyl ether is shown below.
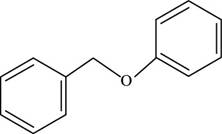
Figure 2
Explanation of Solution
The structure of benzyl phenyl ether is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of benzyl phenyl ether is shown below.

Figure 2
c)
Interpretation:
The structure of benzonitrile is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 46P
The structure of benzonitrile is shown below.
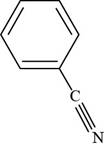
Figure 3
Explanation of Solution
The structure of benzonitrile is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of benzonitrile is shown below.
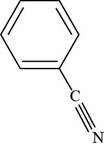
Figure 3
d)
Interpretation:
The structure of benzaldehyde is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 46P
The structure of benzaldehyde is shown below.
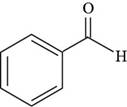
Figure 4
Explanation of Solution
The structure of benzaldehyde is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of benzaldehyde is shown below.
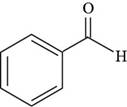
Figure 4
e)
Interpretation:
The structure of anisole is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 46P
The structure of anisole is shown below.
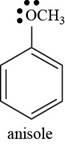
Figure 5
Explanation of Solution
The structure of anisole is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of anisole is shown below.
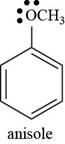
Figure 5
f)
Interpretation:
The structure of styrene is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 46P
The structure of styrene is shown below.
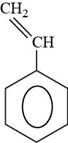
Figure 6
Explanation of Solution
The structure of styrene is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of styrene is shown below.
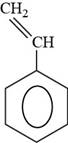
Figure 6
g)
Interpretation: The structure of toluene is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 46P
The structure of toluene is shown below.
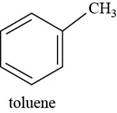
Figure 7
Explanation of Solution
The structure of toluene is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of toluene is shown below.
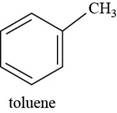
Figure 7
h)
Interpretation: The structure of tert-butyl benzene is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 46P
The structure of tert-butyl benzene is shown below.
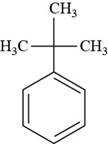
Figure 8
Explanation of Solution
The structure of tert-butyl benzene is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of tert-butyl benzene is shown below.

Figure 8
i)
Interpretation:
The structure of benzyl chloride is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 46P
The structure of benzyl chloride is shown below.
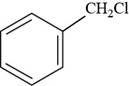
Figure 9
Explanation of Solution
The structure of benzyl chloride is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of benzyl chloride is shown below.

Figure 9
Want to see more full solutions like this?
Chapter 19 Solutions
Organic Chemistry - Access
- Draw structural formulas for organic products A and Barrow_forwardAn unknown compound is believed to be one of these three compounds (benzilic acid, propanoic acid, or cyclohexanomine). Explain how you would go about finding out your compound is ......., using solubility tests? Benzelic acid or propanoic acid or cyclohexanomineAn unknown compound is believed to be one of these three compounds (benzilic acid, propanoic acid, or cyclohexanomine). Explain how you would go about finding out your compound is ......., using solubility tests? Benzelic acid or propanoic acid or cyclohexanominearrow_forward(a) Write a chemical test to distinguish between: (i) Chlorobenzene and Benzyl chloride. (ii) Chloroform and Carbon tetrachloride. (b) Why is methyl chloride hydrolysed more easily than chlorobenzene?arrow_forward
- Explain why a bag of charcoal briquettes contains the following warning: Do not use for indoor heating or cooking unless ventilation is provided for exhausting fumes to the outside.arrow_forwardWhat reagents are used in the esterification of Alcohols and Phenols? a.Write the reaction involved in Primary Alcohol (Ethanol) and Acetyl Chloride b. Write the reaction involved in Phenol and Acetyl Chloridearrow_forwardexplain the following observations a. propanol is more water soluble than propane b. propanol is more water soluble than propanonearrow_forward
- Draw several molecules of methanamide and show the intermolecular hydrogen bonding that account for the amide’s very high boiling point.arrow_forwardDraw the structure of benzaldehyde :arrow_forwardWhat reagents are used in the esterification of Alcohols and Phenols? a. Write the reaction involved in Primary Alcohol (Ethanol) and Acetyl Chloride b. Write the reaction involved in Phenol and Acetyl Chloride What is the purpose of the Chromic acid test? a. What are the reagents used? b. Write oxidation reaction of Primary Alcohols and Secondary Alcoholsarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning


