Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 19.4, Problem 19.2P
Problem 19-2
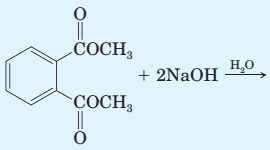
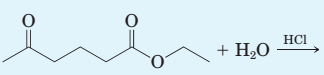
Complete the equation for each hydrolysis reaction. Draw all products as they are ionized under these experimental conditions.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
PROBLEM 22-17Show the products of the reactions of these carboxylic acids with PBr3>Br2 before andafter hydrolysis. ) succinic acid
PROBLEM 20-1Draw the structures of the following carboxylic acids. trans-2-methylcyclohexanecarboxylic acid
PROBLEM 18-9Show how the following transformations may be accomplished in good yield. You mayuse any additional reagents that are needed. benzoic acid S phenyl cyclopentyl ketone
Chapter 19 Solutions
Introduction to General, Organic and Biochemistry
Ch. 19.1 - Prob. 19.1PCh. 19.4 - Problem 19-2 Complete the equation for each...Ch. 19.4 - Prob. 19.3PCh. 19 - Prob. 19.4PCh. 19 - Write the IUPAC name for each compound.Ch. 19 - Prob. 19.6PCh. 19 - Prob. 19.7PCh. 19 - Prob. 19.8PCh. 19 - Prob. 19.9PCh. 19 - 0 Complete the equations for these reactions.
Ch. 19 - Prob. 19.11PCh. 19 - Prob. 19.12PCh. 19 - Prob. 19.13PCh. 19 - Prob. 19.14PCh. 19 - Prob. 19.15PCh. 19 - 6 Why are Dacron and Mylar referred to as...Ch. 19 - 7 What type of structural feature do the...Ch. 19 - Prob. 19.18PCh. 19 - Prob. 19.19PCh. 19 - 0 Show how triphosphoric acid can form from three...Ch. 19 - 1 Write an equation for the hydrolysis of...Ch. 19 - 2 (Chemical Connections 19A) Locate the ester...Ch. 19 - Prob. 19.23PCh. 19 - Prob. 19.24PCh. 19 - Prob. 19.25PCh. 19 - Prob. 19.26PCh. 19 - Prob. 19.27PCh. 19 - 8 (Chemical Connections 19C) Once it has been...Ch. 19 - Prob. 19.29PCh. 19 - Prob. 19.30PCh. 19 - Prob. 19.31PCh. 19 - Prob. 19.32PCh. 19 - Prob. 19.33PCh. 19 - 4 (Chemical Connections 19F) Why do Lactomer...Ch. 19 - Prob. 19.35PCh. 19 - Prob. 19.36PCh. 19 - Prob. 19.37PCh. 19 - 8 In Chapter 22, we will discuss a class of...Ch. 19 - Prob. 19.39PCh. 19 - Prob. 19.40PCh. 19 - Prob. 19.41PCh. 19 - Prob. 19.42PCh. 19 - Prob. 19.43PCh. 19 - Prob. 19.44PCh. 19 - Prob. 19.45PCh. 19 - Prob. 19.46PCh. 19 - Prob. 19.47PCh. 19 - Prob. 19.48PCh. 19 - Prob. 19.49P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Problem 16-4 Select the stronger base from each pair of amines.arrow_forwardPROBLEM 18-28(a) Propose a mechanism for the acid-catalyzed reaction of cyclohexanone with ethyleneglycol to give cyclohexanone ethylene acetal.(b) Propose a mechanism for the acid-catalyzed hydrolysis of cyclohexanone ethylene acetal.(c) Compare the mechanisms you drew in parts (a) and (b). How similar are these mechanisms,comparing them in reverse order?arrow_forwardProblem 4.9 Predict the product. Why?arrow_forward
- Problem 17-6 Show the reaction of benzaldehyde with one molecule of methanol to form a hemiacetal and then with a second molecule of methanol to form an acetal.arrow_forwardPROBLEM 14-27Predict the major products of the following reactions, including stereochemistry whereappropriate.2,2-dimethyloxirane + H18O- >H218Oarrow_forwardWhat advantages and disadvantages of N-formylation? Why do we make it? please 1. abstract 2. introduction 3. methodology 4.result and discuss 5. conclusionarrow_forward
- Condensation Exercises Intrusctions: Solve by condensation (exercise 1). Solve by crossed aldol reactions (exercise 2).arrow_forwardProblem 17-7 Identify all hemiacetals and acetals in the following structures and tell whether each is formed from an aldehyde or a ketone. OH (a) (b) CH3OCH2CH2OCH3 (c)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY