Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter17: Electrochemistry And Its Applications
Section: Chapter Questions
Problem 38QRT
Related questions
Question
100%
Use the tabulated electrode potentials to calculate K for the oxidation of zinc by H+ (at 25 ∘C):
Zn(s)+2H+(aq)→Zn2+(aq)+H2(g)
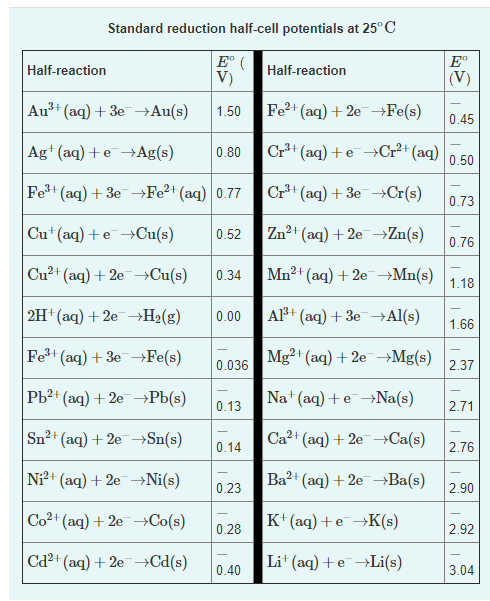
Transcribed Image Text:Standard reduction half-cell potentials at 25° C
E° (
V)
E°
Half-reaction
Half-reaction
|(V)
Au? (aq) + Зе Au('s)
1.50
Fe2 (aq) + 2e-→Fe(s)
0.45
Ag+ (aq) +e→Ag(s)
Cr3+ (aq) +e→Cr²+ (aq)
0.80
0.50
Fe+ (aq) + 3e→Fe?+ (aq) 0.77
Cr" (aq) + 3e-→Cr(s)
0.73
Cu+ (aq) + €¯→CU(s)
Zn2+
(аq) + 2е —Zn(s)
0.52
0.76
Cu2+ (aq) + 2e →Cu(s)
Mn2+ (aq) + 2e →Mn(s)
0.34
1.18
| 2H (aq) + 20→H2(g)
0.00
Al+ (aq) + 3e-→Al(s)
1.66
Fe (aq) + 3e–→Fe(s)
Mg²+ (aq) + 2e–→Mg(s)
0.036
2.37
Pb2+ (aq) + 20→P6(s)
Na+ (aq) + e¯→Na(s)
0.13
2.71
Sn²+ (aq) + 2e→Sn(s)
Ca2+ (aq) + 2e→Ca(s)
0.14
2.76
Ni?+ (aq) + 2e →Ni(s)
Bа? (аq) + 2е —Ba(s)
0.23
2.90
Co2+ (aq) + 20→C0(s)
K* (aq) +e→K(s)
0.28
2.92
Cd2+ (aq) + 2e →Cd(s)
Lit (aq) +E→Lİ(s)
0.40
3.04
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
Expert Answers to Latest Homework Questions
Q: A standard deck of playing cards consists of 52 cards. Each card has a rank and a suit. There are 13…
Q: A standard deck of playing cards consists of 52 cards. Each card has a rank and a suit. There are 13…
Q: A tsunami destroyed Kyoto Company's warehouse and all of its inventory. Kyoto's management believes…
Q: If the tubular shaft is made from material having an
allowable shear stress of Tallow = 90 MPa,…
Q: What is the concentration of A after 10.5 minutes for the reaction A → Products when the initial…
Q: Balance the reaction between NO and Ni²+ to form NO3 and Ni in
acidic solution. When you have…
Q: Find the value of za.
z0.11
Q: Enter electrons as e*.
Use smallest possible integer coefficients for ALL reactions.
If a box is not…
Q: Enter electrons as e".
Use smallest possible integer coefficients.
If a box is not needed, leave it…
Q: Customer
Bad Debt Expense, Aging of Accounts Receivable, Journal Entry.
Giaraldi Garden Products,…
Q: how do i solve
Q: how do i solve
Q: How long will it take for the concentration of A to decrease from 0.500 M to 0.200 M in the…
Q: Required information
A scuba diver is in fresh water has an air tank with a volume of 0.0100
Pa.
m³.…
Q: A 55.7-kg sky diver jumped out of an airplane at an altitude of 0.900 km. She opened her
parachute…
Q: A 9.70-kg steel ball at 15.1°C is dropped from a height of 13.2 m into an insulated container
with…
Q: Question 5
Find f(4) for the recursive function if f(n) is defined recursively by
ƒ(n + 1) = f(n) +…
Q: At the end of each month, for 24 months, $200 is put into an account paying 6% annual interest…
Q: !
Required information
Tina is going to make iced tea by first brewing hot tea, then adding ice…
Q: Find bar CE
Q: what is the pH of 5.00 g of Na2CO3 and 5.00 g of NaHCO3 diluted to 0.100 L