
Concept explainers
a) Bromoacetone
Interpretation:
The structure for bromoacetone is to be shown.
Concept introduction:
To show:
The structure for bromoacetone.
Answer to Problem 54AP
The structure of bromoacetone is
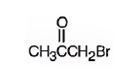
Explanation of Solution
The name indicates that the compound is a ketone with three carbon straight chain with a bromine atom attached to C1.
The structure of bromoacetone is

b) (S)-2-Hydroxypropanal
Interpretation:
The structure for (S)-2-hydroxypropanal is to be shown.
Concept introduction:
To show:
The structure for (S)-2-hydroxypropanal.
Answer to Problem 54AP
The structure of (S)-2-hydroxypropanal is
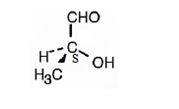
Explanation of Solution
The name indicates that the compound is an aldehyde with three carbon straight chain with a hydroxyl group attached to C2. The molecule is chiral. The three groups, -OH(first highest priority), -CHO (second highest priority) and -CH3(third highest priority) are arranged anticlockwise when viewed from the side away from H (fourth highest priority). Hence it has S stereochemistry.
The structure of (S)-2-hydroxypropanal is

c) 2-Methyl-3-heptanone
Interpretation:
The structure for 2-methyl-3-heptanone is to be shown.
Concept introduction:
Ketones are named by replacing the terminal –e of the parent alkane with –one. The parent chain is the longest one that includes the ketone group and the numbering begins at the end nearer to the carbonyl carbon. If other functional groups are present the double bonded oxygen is considered as a substituent on the parent chain with the prefix –oxo.
To show:
The structure for 2-methyl-3-heptanone.
Answer to Problem 54AP
The structure of 2-methyl-3-heptanone is
Explanation of Solution
The name indicates that the compound is a ketone with seven carbon straight chain having the keto group at position three and a methyl group attached to C2.
The structure of 2-methyl-3-heptanone is
d) (2S,3R)-2,3,4-Trihydroxybutanal
Interpretation:
The structure for (2S,3R)-2,3,4-trihydroxybutanal is to be shown.
Concept introduction:
Aldehydes are named by replacing the terminal –e of the parent alkane with –al. The parent chain is the longest one that includes the -CHO group and the –CHO group is numbered as carbon 1. For cyclic alcohols in which the –CHO group is directly attached to the ring, the suffix –carbaldehyde is used.
To show:
The structure for (2S,3R)-2,3,4-trihydroxybutanal.
Answer to Problem 54AP
The structure of (2S,3R)-2,3,4-trihydroxybutanal is
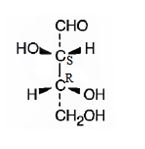
Explanation of Solution
The name indicates that the compound is an aldehyde with four carbon straight chain and has three hydroxyl groups attached to C2, C3 and C4.
The molecule is chiral. The C2 is attached to the three groups, -OH(first highest priority), -CHO (second highest priority) and –C3 (third highest priority) arranged anticlockwise when viewed from the side away from H (fourth highest priority). Hence it has S stereochemistry.
The C3 is attached to the three groups, -OH(first highest priority), –C2 (second highest priority) and –CH2OH--(third highest priority) arranged clockwise when viewed from the side away from H (fourth highest priority). Hence it has R stereochemistry.
The structure of (2S,3R)-2,3,4-trihydroxybutanal is

e) 2,2,4,4-Tetramethyl-3-pentanone
Interpretation:
The structure for 2,2,4,4-tetramethyl-3-pentanone is to be shown.
Concept introduction:
Ketones are named by replacing the terminal –e of the parent alkane with –one. The parent chain is the longest one that includes the ketone group and the numbering begins at the end nearer to the carbonyl carbon. If other functional groups are present the double bonded oxygen is considered as a substituent on the parent chain with the prefix –oxo.
To show:
The structure for 2,2,4,4-tetramethyl-3-pentanone.
Answer to Problem 54AP
The structure of 2,2,4,4-tetramethyl-3-pentanone is
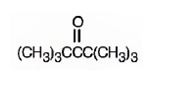
Explanation of Solution
The name indicates that the compound is a ketone with five carbon straight chain with a keto group at position three attached to four methyl groups, two on C2 and other two on C4.
The structure of 2,2,4,4-Tetramethyl-3-pentanone is
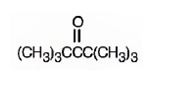
f) 4-methyl-3-penten-2-one
Interpretation:
The structure for 4-methyl-3-penten-2-one is to be shown.
Concept introduction:
Ketones are named by replacing the terminal –e of the parent alkane with –one. The parent chain is the longest one that includes the ketone group and the numbering begins at the end nearer to the carbonyl carbon. If other functional groups are present the double bonded oxygen is considered as a substituent on the parent chain with the prefix –oxo.
To show:
The structure for 4-methyl-3-penten-2-one.
Answer to Problem 54AP
The structure of 4-methyl-3-penten-2-one is
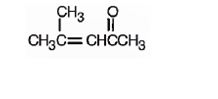
Explanation of Solution
The name indicates that the compound is a ketone containing a five carbon straight chain, having a keto group at position two and a double bond between C3 and C4 with a methyl group on C4.
The structure of 4-methyl-3-penten-2-one is

g) Butanedial
Interpretation:
The structure for butanedial is to be shown.
Concept introduction:
Aldehydes are named by replacing the terminal –e of the parent alkane with –al. The parent chain is the longest one that includes the -CHO group and the –CHO group is numbered as carbon 1. For cyclic alcohols in which the –CHO group is directly attached to the ring, the suffix –carbaldehyde is used.
To show:
The structure for butanedial.
Answer to Problem 54AP
The structure of butanedial is
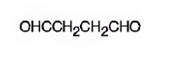
Explanation of Solution
The name of the compound indicates that it has a four carbon straight chain with two aldehyde groups at both ends.
The structure of butanedial is

h) 3-Phenyl-2-propenal
Interpretation:
The structure for 3-phenyl-2-propenal is to be shown.
Concept introduction:
Aldehydes are named by replacing the terminal –e of the parent alkane with –al. The parent chain is the longest one that includes the -CHO group and the –CHO group is numbered as carbon 1. For cyclic alcohols in which the –CHO group is directly attached to the ring, the suffix –carbaldehyde is used.
To show:
The structure for 3-phenyl-2-propenal.
Answer to Problem 54AP
The structure of 3-phenyl-2-propenal is
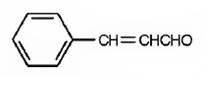
Explanation of Solution
The name of the compound indicates that the compound is a three carbon aldehyde with a double bond between C2 & C3 and has a phenyl group attached to C3.
The structure of 3-phenyl-2-propenal is

i) 6,6-Dimethyl-2,4-cyclohexadienone
Interpretation:
The structure for 6,6-dimethyl-2,4-cyclohexadienone is to be shown.
Concept introduction:
Ketones are named by replacing the terminal –e of the parent alkane with –one. The parent chain is the longest one that includes the ketone group and the numbering begins at the end nearer to the carbonyl carbon. If other functional groups are present the double bonded oxygen is considered as a substituent on the parent chain with the prefix –oxo.
To show:
The structure for 6,6-dimethyl-2,4-cyclohexadienone.
Answer to Problem 54AP
The structure of 6,6-dimethyl-2,4-cyclohexadienone is
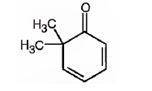
Explanation of Solution
The name of the compound indicates that it is a cyclic ketone with a cyclohexadiene ring containing two double bonds, one between C2 & C3 and other between C4 & C5. It also has two methyl groups on C6.
The structure of 6,6-dimethyl-2,4-cyclohexadienone is

j) p-Nitroacetophenone
Interpretation:
The structure for p-nitroacetophenone is to be shown.
Concept introduction:
Ketones are named by replacing the terminal –e of the parent alkane with –one. The parent chain is the longest one that includes the ketone group and the numbering begins at the end nearer to the carbonyl carbon. If other functional groups are present the double bonded oxygen is considered as a substituent on the parent chain with the prefix –oxo. Some common names like acetophenone are retained by IUPAC.
To show:
The structure for p-nitroacetophenone.
Answer to Problem 54AP
The structure of p-nitroacetophenone is
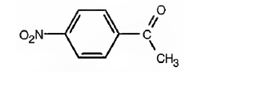
Explanation of Solution
The name of the compound indicates that it contains an actyl and nitro groups attached to a benzene ring in para relationship.
The structure of p-nitroacetophenone is

Want to see more full solutions like this?
Chapter 19 Solutions
Organic Chemistry
- Draw structures corresponding to the following names: (a) 3-Methyl-1, 2-benzenediamine (b) 1, 3, 5-Benzenetriol (c) 3-Methyl-2-phenylhexane (d) o-Aminobenzoic acid (e) m-Bromophenol (f) 2, 4, 6-Trinitrophenol (picric acid]arrow_forwardDraw the products formed when p-methylaniline (p-CH3C6H4NH2) istreated with following reagent. Part (b), then CH3COCl, AlCl3arrow_forwardGive the structure corresponding to each name: (a) sec-butyl ethyl ketone; (b) methyl vinyl ketone; (c) pethylacetophenone; (d) 3-benzoyl-2-benzylcyclopentanone; (e) 6,6-dimethylcyclohex-2-enone; (f) 3-ethylhex-5-enal.arrow_forward
- The IUPAC name of the CORRECT answer in Q45 above is? a. 4-Pyridyl phenyl ketone b. Diphenyl ketone c. Cyclohexyl phenyl ketone d. Cyclohexyl benzoatearrow_forward5-Hydroxyhexanal forms a six-membered cyclic hemiacetal, which predominates at equilibrium in aqueous solution. (a) Draw a structural formula for this cyclic hemiacetal. (b) How many stereoisomers are possible for 5-hydroxyhexanal? (c) How many stereoisomers are possible for this cyclic hemiacetal? (d) Draw alternative chair conformations for each stereoisomer and label groups axial or Also predict which of the alternative chair conformations for each stereoisomer is more stable.arrow_forwardDraw the products formed when p-methylaniline (p-CH3C6H4NH2) is treated with each reagent. a. HCl b. CH3COCl c. (CH3CO)2O d. excess CH3I e. (CH3)2C = O f. CH3COCl, AlCl3 g. CH3CO2H h. NaNO2, HCl i. Part (b), then CH3COCl, AlCl j. CH3CHO, NaBH3CNarrow_forward
- Draw the structure corresponding to each name.a. 3,3-dimethylpentanoic acidb. 4-chloro-3-phenylheptanoic acidc. (R)-2-chloropropanoic acidd. m-hydroxybenzoic acide. potassium acetatef. sodium α-bromobutyrateg. 2,2-dichloropentanedioic acidh. 4-isopropyl-2-methyloctanedioic acidi. 3,3-dimethylpentanenitrile j. 4,5-diethyl-2-isopropylnonanenitrilearrow_forward3. Draw the structure of the following compounds: e.1-methyl(N-ethyl-N-methyl)propenamidef. Butanoic anhydrideg. 4-Methylhex-3-ene-2-thiolh. cis-but-2-enedioic acidi. acetaminophenarrow_forwardDraw the structure corresponding to each name.a. 3,3-dimethylpentanoic acidb. 4-chloro-3-phenylheptanoic acidc. (R)-2-chloropropanoic acidd. m-hydroxybenzoic acide. potassium acetatef. sodium a-bromobutyrateg. 2, 2-dichloropentanedioic acidh. 4-isopropyl-2-methyloctanedioic acidarrow_forward
- Answer the following questions about 2-acetylcyclopentanone.a. What starting materials are needed to form 2-acetylcyclopentanone by a Claisen reaction that forms bond (a)?b. What starting materials are needed to form 2-acetylcyclopentanone by a Claisen reaction that forms bond (b)?c. What product is formed when 2-acetylcyclopentanone is treated with NaOCH2CH3, followed by CH3I?d. Draw the Robinson annulation product(s) formed by reaction of 2- acetylcyclopentanone with methyl vinyl ketone (CH2=CHCOCH3).e. Draw the structure of the most stable enol tautomer(s).arrow_forwardHow do you separate a mixture of naphthalene, biphenyl-4-carboxylic acid, and 4-chloroaniline? Thank you!arrow_forwardUsing ChemDraw Draw a mechanism of a reversible reaction of acetophenone and strong acid (H-A) to form a protonated acetophenone and a weak base. Draw all the electron pairs of the molecules and the arrows.arrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

