
a)
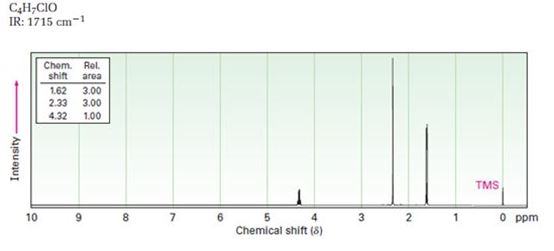
Interpretation:
A structure for ketone or aldehyde with the following descriptions is to be proposed.
C4H7ClO, IR: 1715 cm-1; 1HNMR: 1.62 δ (Rel.area=3.00), 2.33 δ (Rel.area=3.00), 4.32δ (Rel.area=1.00).
Concept introduction:
In 1HNMR the aldehyde protons absorb near 10 δ with a coupling constant , J = 3Hz. Hydrogens on the carbon next to aldehyde group absorb near 2.0-2.3 δ. Methyl ketones show a sharp three proton singlet near 2.1 δ.
To purpose:
A structure for ketone or aldehyde with the following descriptions.
C4H7ClO, IR: 1715 cm-1; 1HNMR: 1.62 δ (Rel.area=3.00), 2.33 δ (Rel.area=3.00), 4.32δ (Rel.area=1.00).
b)
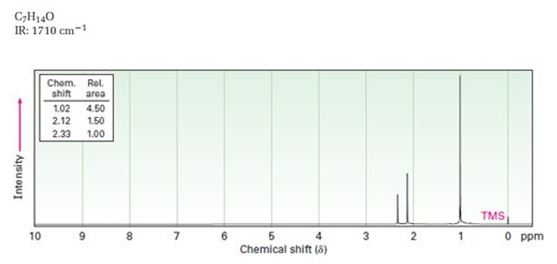
Interpretation:
A structure for ketone or aldehyde with the following descriptions is to be proposed.
C7H14O, IR: 1710 cm-1; 1HNMR: 1.02 δ (Rel.area=4.50), 2.12 δ (Rel.area=1.50), 2.33δ (Rel.area=1.00).
Concept introduction:
Aldehydes and ketones show a strong absorption band in IR from 1660-1770 cm-1. Aldehydes show two characteristic C-H absorptions between 2700-2760 cm-1 and 2800-2860 cm-1. Saturated aldehydes absorb near 1730 cm-1 while aromatic aldehydes and α, β- unsaturated aldehydes absorb near 1705 cm-1. Saturated ketones and cyclohexanones absorb near 1715 cm-1 while aromatic ketones and α, β- unsaturated ketones absorb near 1685-1690 cm-1. Cyclopentanones absorb around 1750 cm-1.
In 1HNMR the aldehyde protons absorb near 10 δ with a coupling constant , J = 3Hz. Hydrogens on the carbon next to aldehyde group absorb near 2.0-2.3 δ. Methyl ketones show a sharp three proton singlet near 2.1 δ.
To purpose:
A structure for ketone or aldehyde with the following descriptions.
C7H14O, IR: 1710 cm-1; 1HNMR: 1.02 δ (Rel.area=4.50), 2.12 δ (Rel.area=1.50), 2.33δ (Rel.area=1.00).
Want to see the full answer?
Check out a sample textbook solution
Chapter 19 Solutions
Organic Chemistry
- Propose structures for alcohols that have the following 1HNMR spectra: (a) C5H12Oarrow_forwardTreatment of 1-aminoadamantane, C10H17N, with methyl 2,4-dibromobutanoate in the presence of a nonnucleophilic base, R3N, involves two successive SN2 reactions and gives compound A. Propose a structural formula for compound A.arrow_forwardThe 1H and 13C NMR spectra below belong to a compound with formula C6H10O2. Propose a structure for this compound.arrow_forward
- Show how each of the following compounds can be synthesized from benzene: a. o-bromopropylbenzene b. m-nitrobenzoic acid c. p-nitrobenzoic acid d. p-chloroaniline e. m-chloroaniline f. m-xylene g. 2-phenylpropene h. m-bromopropylbenzene i. 1-phenyl-2-propanolarrow_forwardCompound B of molecular formula C9H19N shows a noteworthy infrared absorption at 3300 cm-1. Its 1H-NMR spectrum shows three singlets – δ 1.0 (6H), 1.1 (12H), 1.4 (1H) ppm. Its 13C-NMR spectrum has four signals – δ 25, 28, 41, 64 ppm. Suggest a structure for this compound. Please show work.arrow_forwardPropose the structure of the following alcohol? Molecular formula C5H12O The following peaks are found in proton NMR spectrum 0.91 δ (3H, triplet) 1.19 δ (6Η, singlet) 1.50 δ (2H, quartet) 2.0 δ (1H, singlet)arrow_forward
- Using cyclohexanone as the starting material, describe how the following compounds can be synthesized:arrow_forwardPropose a structure for compound X (molecular formula C6H12O2), which gives a strong peak in its IR spectrum at 1740 cm−1. The 1H NMR spectrum of X shows only two singlets, including one at 3.5 ppm. The 13C NMR spectrum is given below. Propose a structure for X.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

