
a)
Interpretation:
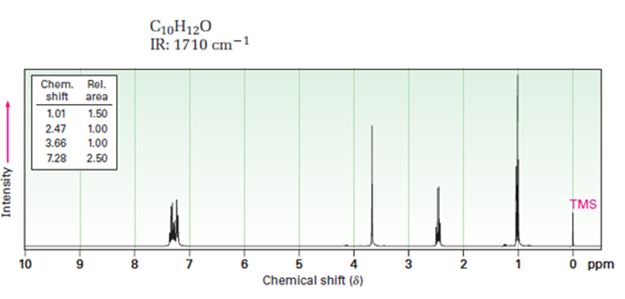
A structure for ketone or aldehyde with the following descriptions is to be proposed.
C10H12O, IR: 1710 cm-1; 1HNMR: 1.01 δ (Rel.area=1.50), 2.47 δ (Rel.area=1.00), 3.66 δ (Rel.area=1.00), 7.28 δ (Rel.area=2.50).
Concept introduction:
In 1HNMR the aldehyde protons absorb near 10 δ with a coupling constant, J=3Hz. Hydrogens on the carbon next to aldehyde group absorb near 2.0-2.3 δ. Methyl ketones show a sharp three proton singlet near 2.1 δ.
To propose:
A structure for ketone or aldehyde with the following descriptions.
C10H12O, IR: 1710 cm-1; 1HNMR: 1.01 δ (Rel.area=1.50), 2.47 δ (Rel.area=1.00), 3.66 δ (Rel.area=1.00), 7.28 δ (Rel.area=2.50).
b)
Interpretation:
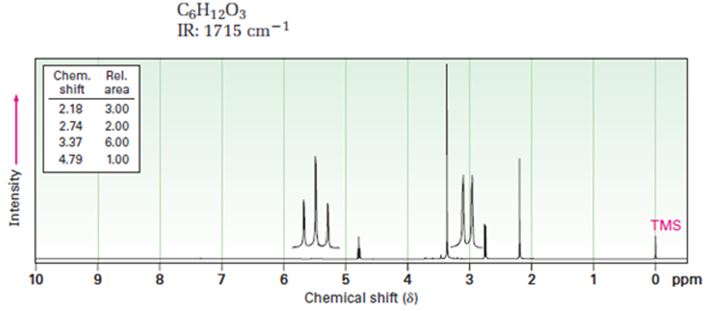
A structure for ketone or aldehyde with the following descriptions is to be proposed.
C6H12O3, IR: 1715 cm-1; 1HNMR: 2.18 δ (Rel.area=3.00), 2.74 δ (Rel.area=2.00), 3.37 δ (Rel.area=6.00), 4.79 δ (Rel.area=1.00).
Concept introduction:
Aldehydes and ketones show a strong absorption band in IR from 1660-1770 cm-1. Aldehydes show two characteristic C-H absorptions between 2700-2760 cm-1 and 2800-2860 cm-1. Saturated aldehydes absorb near 1730 cm-1 while aromatic aldehydes and α, β-unsaturated aldehydes absorb near 1705 cm-1. Saturated ketones and cyclohexanones absorb near 1715 cm-1 while aromatic ketones and α, β-unsaturated ketones absorb near 1685-1690 cm-1. Cyclopentanones absorb around 1750 cm-1.
In 1HNMR the aldehyde protons absorb near 10 δ with a coupling constant, J=3Hz. Hydrogens on the carbon next to aldehyde group absorb near 2.0-2.3 δ. Methyl ketones show a sharp three proton singlet near 2.1 δ.
To propose:
A structure for ketone or aldehyde with the following descriptions.
C6H12O3, IR: 1715 cm-1; 1HNMR: 2.18 δ (Rel.area=3.00), 2.74 δ (Rel.area=2.00), 3.37 δ (Rel.area=6.00), 4.79 δ (Rel.area=1.00).
Trending nowThis is a popular solution!

Chapter 19 Solutions
Organic Chemistry
- Propose structures for alcohols that have the following 1HNMR spectra: (a) C5H12Oarrow_forwardTreatment of 1-aminoadamantane, C10H17N, with methyl 2,4-dibromobutanoate in the presence of a nonnucleophilic base, R3N, involves two successive SN2 reactions and gives compound A. Propose a structural formula for compound A.arrow_forwardThe 1H and 13C NMR spectra below belong to a compound with formula C6H10O2. Propose a structure for this compound.arrow_forward
- Show how the following compounds can be synthesized from benzene: a. m-chlorobenzenesulfonic acid d. 1-phenylpentane g. p-cresol b. m-chloroethylbenzene e. m-bromobenzoic acid h. benzyl alcohol c. m-bromobenzonitrile f. m-hydroxybenzoic acid i. benzylaminearrow_forwardAn unknown compound has a molecular formula of C3H6O2. Its IR spectrum shows a very strong and broad band at 2980 and a strong sharp peak at 1716 cm-1. It exhibits the following signals in its 1H NMR spectrum (ppm): 1.21 (triplet, 3H), 2.48 (quartet, 2H), 11.7 (singlet, 1H); and the following signals in its 13C NMR spectrum (ppm): 8.9, 27.6, 181.5. Draw the structure of the unknown compound.arrow_forwardPlease propose structures for ketones or aldehydes having the following 1H NMR spectraarrow_forward
- Adiol (C8H1802) does not react with periodic acid. Its 1H NMR spectrum contains three singlets at § 1.2 (12 protons), 1.6 (4 protons), and 2.0 ppm (2 protons). What is the structure of this diol?arrow_forwardPropose the structure of the following alcohol? Molecular formula C5H12O The following peaks are found in proton NMR spectrum 0.91 δ (3H, triplet) 1.19 δ (6Η, singlet) 1.50 δ (2H, quartet) 2.0 δ (1H, singlet)arrow_forwardPropose a structure for an alcohol with molecular formula C5H12O that has the 1H NMR spectrum given below. Given chemical shifts and splitting patterns: 0.95 ppm (3H, triplet) 1.3 ppm (6H, singlet) 1.5 ppm (2H, quartet) 2.0 ppm (1H, singlet)arrow_forward
- Show how the following compounds can be synthesized from benzene: a. p-nitrobenzoic acid c. o-chlorophenol e. p-methylbenzonitrile b. m-bromophenol d. m-methylnitrobenzene f. m-chlorobenzaldehydearrow_forwardCompound B of molecular formula C9H19N shows a noteworthy infrared absorption at 3300 cm-1. Its 1H-NMR spectrum shows three singlets – δ 1.0 (6H), 1.1 (12H), 1.4 (1H) ppm. Its 13C-NMR spectrum has four signals – δ 25, 28, 41, 64 ppm. Suggest a structure for this compound. Please show work.arrow_forwardShow how m-toluidine can be converted tom-toluidineCH3 NH2the following compounds, using any necessary reagents.m-toluonitrile(a) CH3 C N CH3 CH2NH2m-methylbenzylamine(b)m-iodotolueneCH I 3 (c)m-cresol(d) CH3 OH3-methyl-4-nitroanilineCH3 NH2O2N(e)NHCH3N-cyclopentyl-m-toluidinearrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

