
Biochemistry
6th Edition
ISBN: 9781305577206
Author: Reginald H. Garrett, Charles M. Grisham
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 15P
Answers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book.
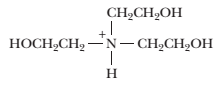
How to Prepare a Triethanolamine Buffer Shown here is the structure of triethanolamine in its fully protonated form:
Its pKa is 7.8. You have available at your kb bench 0.1 M solutions of HO, NaOH, and the uncharged (free base) form of triethanolamine, as well as ample distilled water. Describe the preparation of a 1-L solution of 0.05 M triethanolamine buffer, pH 7.6.
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 2 Solutions
Biochemistry
Ch. 2 - Answers to all problems are at the end of this...Ch. 2 - Answers to all problems are at the end of this...Ch. 2 - Answers to all problems are at the end of this...Ch. 2 - Answers to all problems are at the end of this...Ch. 2 - Answers to all problems are at the end of this...Ch. 2 - Answers to all problems are at the end of this...Ch. 2 - Answers to all problems are at the end of this...Ch. 2 - Answers to all problems are at the end of this...Ch. 2 - Answers to all problems are at the end of this...Ch. 2 - Answers to all problems are at the end of this...
Ch. 2 - Answers to all problems are at the end of this...Ch. 2 - Answers to all problems are at the end of this...Ch. 2 - Answers to all problems are at the end of this...Ch. 2 - Prob. 14PCh. 2 - Answers to all problems are at the end of this...Ch. 2 - Prob. 16PCh. 2 - Answers to all problems are at the end of this...Ch. 2 - Prob. 18PCh. 2 - Answers to all problems are at the end of this...Ch. 2 - Answers to all problems are at the end of this...Ch. 2 - Answers to all problems are at the end of this...Ch. 2 - Prob. 22PCh. 2 - Answers to all problems are at the end of this...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Answers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Draw the Titration Curve for a Weak Acid and Determine its pKa from the Titration Curve When a 0.1 M solution of a weak acid was titrated with base, the following results were obtained: Plot the results of this titration and determine the pK a of the weak acid from your graph.arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Interpreting Kinetics Experiments from Graphical Patterns The following graphical patterns obtained from kinetic experiments have several possible interpretations depending on the nature of the experiment and the variables being plotted. Give at least two possibilities for each.arrow_forwardAnswers to all problems are at the end of this book Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Solving the Sequence of an Oligopeptide From Sequence Analysis Data Analysis of the blood of a catatonic football fan revealed large concentrations of a. psychologic octapeptide. Amino acid analysis of this oclapeplide gave the following results: 2 Ala lArg 1 Asp 1 Mel 2 Tyr I Val 1NH/ The following facts were observed: Partial acid hydrolysis of the octapeptide yielded a dipeptide of the structure Chymolrypsin treatment of the octapeplide yielded two tetrapeptides, each containing an alanine residue. Trypsin treatment of one of the tetrapeptides yielded two dipeptides. Cyanogen bromide treatment of another sample of the same tetrapeplide yielded a tripeplideand free Tyr. N-lerminal analysis of the other tetrapeptide gave Asn. What is the amino acid sequence of this oclapeplide?arrow_forward
- Answers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Preparing a Phosphate Buffer Solution of pH 7.5 from Solutions of Na3PO4 and H3PO4 Given 0.1 M solutions of Na3PO4, and H3PO4 describe the preparation of 1 L of a phosphate buffer at a pH of 7.5. What are the molar concentrations of the ions in the final buffer solution, including Na+ and H+?arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Determining the Branch Points and Reducing Ends of Amylopectin A 0.2-g sample of amylopectin was analyzed to determine the fraction of the total glucose residues, that are branch points in the structure. The sample was exhaustively methylated and then digested, yielding 50-mol of 2,3-dimethylgluetose and 0.4 mol of 1,2,3,6- letramethylglucose. What fraction of the total residues are branch points? I low many reducing ends does this sample of amylopectin have?arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Calculating [H+] from pH Calculate the following from the pH values given in Table 2.3. [H+] in vinegar [H+] in saliva [H+] in household ammonia [OH-] in milk of magnesia [OH-] in beer [H+] inside a liver cellarrow_forward
- Answers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Calculating the pH of a Solution of a Weak Acid; Calculating the pH of the Solution after the Addition of Strong Base The ka for formic acid is 1.78 10-4 M. What is the pH of a 0.1 M solution of formic acid? 150 mL of 0.1 M NaOH is added to 200 mL of 0.1 M formic acid, and water is added to give a final volume of 1 L. What is the pH of the final solution?arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Solving the Sequence of an Oligopeptide From Sequence Analysis Data Amino acid analysis of ail oligopeptide seven residues long gave The following fads were observed: a. Trypsin treatment had no apparent effect. b. The phenylthiohydantoin released by Lid mini degradation was c. Brief chymotrypsin treatment yielded several products, including a dipeptide and a tetrapeptide. The amino acid composition of the tetrapeptide was Leu, Lyi. and Met. d. Cyanogen bromide treatment yielded a dipeptide, a tetrapeptide, and free Lys. What is the amino acid sequence of this heptapeptide?arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. A Rule of Thumb for Amino Acid Content in Proteins The simple average molecular weight of the 20 common amino adds is 138, but most biochemists use 110 when estimating the number of amino acids in a protein of known molecular weight. Why do you Suppose this is? (Hint: There are two contributing factors to the answer. One of them will be apparent from a brief consideration of the amino acid compositions of common proteins. See, for example, Figure 5.16 of this text.)arrow_forward
- Answers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Assessing the pH Dependence of Poly-L-Glutamate Structure Poly-L glutamate adopts an tr-helical structure at low pH but becomes a random coil above pH 5. Explain this behavior.arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Prepare a Buffer by Combining a Solution of Weak Acid with a Solution of the Salt of the Weak Acid Given 0.1 M solutions of acetic acid and sodium acetate, describe the preparation of 1 L of 0.1 M acetate buffer at a pH of 5.4.arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Assessing the Effect of Temperature on Equilibrium You are studying the various components of the venom of a poisonous lizard. One of the venom components is a protein that appears to be temperature sensitive. When heated, it denatures and is no longer toxic. The process can be described by the following simple equation: There is only enough protein from this venom to carry out two equilibrium measurements. At 298 K, you find that 98% of the protein is in its to.\ic form. However, when you raise the temperature to 320 �.. you find that only 10% of the protein is in its toxic form. Calculate the equilibrium constants for the T to N conversion at these two temperatures. Use the data to determine the H,S, and G for this process.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning
Chapter 7 - Human Movement Science; Author: Dr. Jeff Williams;https://www.youtube.com/watch?v=LlqElkn4PA4;License: Standard youtube license