
Modified Mastering Chemistry with Pearson eText -- Standalone Access Card -- for Biochemistry: Concepts and Connections
1st Edition
ISBN: 9780133882797
Author: Dean R. Appling, Spencer J. Anthony-Cahill, Christopher K. Mathews
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 2, Problem 16P
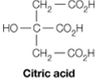
It is possible to make a buffer that functions well near pH 7 using citric acid, which contains only carboxylate groups. Explain
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
It is possible to make a buffer that functions well near pH7 using citric acid, which contains only carboxylate groups. Explain.
We usually say that a perfect buffer has its pH equal to its pKa. Give an example of a situation in which it would be advantageous to have a buffer with a pH 0.5 unit higher than its pKa.
Based on the information in Table 3.2, is there any amino acid that could serve as a buffer at pH 8? If so, which one?
Chapter 2 Solutions
Modified Mastering Chemistry with Pearson eText -- Standalone Access Card -- for Biochemistry: Concepts and Connections
Ch. 2 - Suppose a chloride ion and a sodium ion are...Ch. 2 - Draw two different possible hydrogen-bonding...Ch. 2 - Prob. 3PCh. 2 - 4. What is the pH of each of the following...Ch. 2 - Prob. 5PCh. 2 - The weak acid HA is 2% ionized (dissociated) in a...Ch. 2 - 7. Calculate the pH values and draw the titration...Ch. 2 - What is the pH of the following buffer mixtures?...Ch. 2 - a. Suppose you wanted to make a buffer of exactly...Ch. 2 - Prob. 10P
Ch. 2 - You need to make a buffer whose pH is 7.0, and you...Ch. 2 - Describe the preparation of 2.00 L of 100 glycine...Ch. 2 - Carbon dioxide is dissolved in blood (pH 7.4) to...Ch. 2 - What is the molecular basis for the observation...Ch. 2 - The anno acid arginine ionizes according to the...Ch. 2 - It is possible to make a buffer that functions...Ch. 2 - A student is carrying out a biological preparation...Ch. 2 - Histidine is an amino acid with three titratable...Ch. 2 - Prob. 19PCh. 2 - Prob. 20PCh. 2 - Prob. 21P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Using DEAE-cellulose as ion exchange resin, indicate the starting and ending pH for the narrowest experimental pH range used to separate an amino acid mixture consisting of Gln, Leu and Lys Starting pH: _____ Ending pH: _____arrow_forwardGiven a stock protein solution with a concentration of 6 mg/ml, determine the protein concentration of a solution made by mixing 5 μl of the stock with 5 μl of a buffer.arrow_forwardGiven an 8 M potassium chloride stock solution, explain whether it is possible to perform a dilution resulting in an 11 M working solution.arrow_forward
- What is the ratio of TRIS/TRIS-H+ in a TRIS buffer at pH 8.7?arrow_forwardAn amino acid mixture of phenylalanine, glycine and glutamic acid is to be separated by paper chromatography.The solvent is less polar than water. Which of these amino acids will have the highest Rf value and which the lowest? Explain.arrow_forwardAn amino acid mixture of phenylalanine, glycine and glutamic acid is to be separated by paper chromatography. The solvent is less polar than water. Which of these amino acids will have the highest Rf value and which the lowest? Explain.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you



GCSE Chemistry - Acids and Bases #34; Author: Cognito;https://www.youtube.com/watch?v=vt8fB3MFzLk;License: Standard youtube license