
Identify the elements used in each example of molecular art.
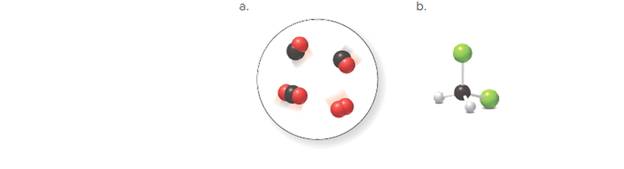
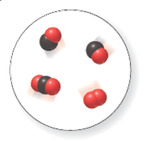
(a)
Interpretation:
The elements present in the following molecular art should be determined:
Concept Introduction:
Molecular art is defined as the art that showcases molecules as physical objects that have a definite shape and size. According to molecular art, some of the atoms are represented with a particular color as follows:
A carbon atom is represented by a black colored sphere.
The oxygen atom is represented by a red-colored sphere.
The nitrogen atom is represented by a blue-colored sphere.
The hydrogen atom is represented by a white-colored sphere.
Chlorine atom is represented by a green-colored sphere.
Bromine atom is represented by a brown-colored sphere.
Answer to Problem 17P
There are two molecules of
Explanation of Solution
In the given space-filling model, there are black and red spheres. Black spheres correspond to the element carbon whereas red spheres correspond to the element oxygen.
Therefore, two molecules contain one carbon atom and one oxygen atom each. Hence, its molecular formula is
Another, one molecule contains one carbon and two oxygen atoms. Hence, its molecular formula is
Another molecule contains only two oxygen atoms. Therefore, its molecular formula is
(b)
Interpretation:
The elements present in the following molecular art should be determined:
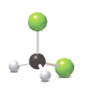
Concept Introduction:
Molecular art is defined as the art that shows molecules as physical objects that have a definite shape and size. According to the molecular art, some of the atoms are represented with a particular color as follows.
A carbon atom is represented by a black colored sphere.
The oxygen atom is represented by a red-colored sphere.
The nitrogen atom is represented by a blue-colored sphere.
The hydrogen atom is represented by a white-colored sphere.
Chlorine atom is represented by a green-colored sphere.
Bromine atom is represented by a brown-colored sphere.
Answer to Problem 17P
The given model contains two chlorine atoms, two hydrogen atoms and one carbon atom. So, elements present in this molecular art are carbon, hydrogen and chlorine.
Explanation of Solution
The given representation of the ball and stick model shows two green spheres, two white spheres and one black sphere.
As it is known that green color represents chlorine element, white color represents the hydrogen element and black color represents carbon element, the molecular formula in the given model is
Hence, it contains two chlorine atoms, two hydrogen atoms and one carbon atom.
Want to see more full solutions like this?
Chapter 2 Solutions
General, Organic, and Biological Chemistry - 4th edition
Additional Science Textbook Solutions
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
Organic Chemistry
Principles of Chemistry: A Molecular Approach (3rd Edition)
Chemistry: The Molecular Nature of Matter
CHEMISTRY-TEXT
Organic Chemistry (9th Edition)
- Give the systematic name for the following compounds that are found in everyday life: a. H2S (rotten egg smell) b. SO2 (smell of burnt matches) c. SFs (aerosol can propellant) d. Na2SO3 (dried fruit preservative)arrow_forwardLook around you and identify several objects that you think are probably made from polymers.arrow_forwardHow many metals are in the following groups? (a) Group 1 (b) Group 13 (c) Group 17arrow_forward
- When water boils, you can see bubbles rising to the surface of the water. Of what arc these bubbles made? air hydrogen and oxygen gas oxygen gas water vapor carbon dioxide gasarrow_forwardSketch a magnified view (showing atoms and/or molecules) of each of the following, and explain why the specified type of mixture is a heterogeneous mixture of two different compounds. a homogeneous mixture of an element and a compound.arrow_forwardRead The Molecular Revolution" box in this chapter on seeing atoms. How is the technology explained in the box a confirmation of the atomic theory? Does the technology "prove the atomic theory?arrow_forward
- a Which of the following substances would you expect to be elements and which would you expect to be compounds? 1 aluminum sulfate; 2 osmium; 3 radon; 4 lithium carbonate; 5 dimethylhydrazine. b On what general rule do you base your answers to part a? Can you name any exceptions to this general rule for compounds?arrow_forward2.94 Use a molecular level description to distinguish between LDPE and HDPE.arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





