
BIOCHEMISTRY BOOKS ALC&MOD MST/ET PKG
1st Edition
ISBN: 9780134172507
Author: APPLING
Publisher: Pearson Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 18P
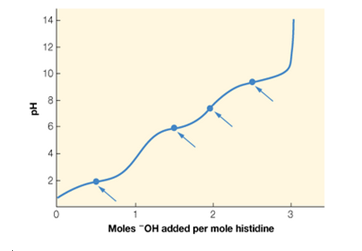
Histidine is an amino acid with three titratable groups: an -NH3+ group (pKa = 9.2) —COOH group (pKa = 1.8). and an imidazole (
a. Identify which point on the titration curve corresponds to the pKa for each of the titratable groups, and which point corresponds to the pl. Explain your choices.
b. Calculate the value of pl for histidine.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Histidine is an amino acid with three titratable groups: an -NH3+ group (pKa = 9.2), a -COOH group (pKa = 1.8), and an imidazole (amine-like) group (pKa = 6.0). The titration curve for histidine is shown below with four points highlighted. (a) Identify which point on the titration curve corresponds to the pKa for each of the titratable groups, and which point corresponds to the pI. Explain your choices. (b) Calculate the value of pI for histidine
Explain why the indole nitrogen of tryptophan is more weakly basic than one of the imidazole nitrogens of histidine
What is the relationship between (R)-cysteine and (S)-alanine
Do they have the opposite three-dimensional configuration (as the names might suggest) or the same configuration?
Is (R)-cysteine a D-amino acid or an L-amino acid?
The common naturally occurring form of cysteine has a chirality center that is named (R), however;
(a) What is the relationship between (R)-cysteine and (S)-alanine?
(b) Do they have the opposite three-dimensional configuration (as the names might suggest) or the same configuration?
(c) Is (R)-cysteine a D-amino acid or an L-amino acid?
Chapter 2 Solutions
BIOCHEMISTRY BOOKS ALC&MOD MST/ET PKG
Ch. 2 - Suppose a chloride ion and a sodium ion are...Ch. 2 - Draw two different possible hydrogen-bonding...Ch. 2 - Prob. 3PCh. 2 - 4. What is the pH of each of the following...Ch. 2 - Prob. 5PCh. 2 - The weak acid HA is 2% ionized (dissociated) in a...Ch. 2 - 7. Calculate the pH values and draw the titration...Ch. 2 - What is the pH of the following buffer mixtures?...Ch. 2 - a. Suppose you wanted to make a buffer of exactly...Ch. 2 - Prob. 10P
Ch. 2 - You need to make a buffer whose pH is 7.0, and you...Ch. 2 - Describe the preparation of 2.00 L of 100 glycine...Ch. 2 - Carbon dioxide is dissolved in blood (pH 7.4) to...Ch. 2 - What is the molecular basis for the observation...Ch. 2 - The anno acid arginine ionizes according to the...Ch. 2 - It is possible to make a buffer that functions...Ch. 2 - A student is carrying out a biological preparation...Ch. 2 - Histidine is an amino acid with three titratable...Ch. 2 - Prob. 19PCh. 2 - Prob. 20PCh. 2 - Prob. 21P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Histidine: draw the amino acid side chain and give the single and three letter abbreviation.arrow_forwardFollowing are two structural formulas for (S)-serine, one of the building blocks of proteins Is (S)-serine better represented by structural formula A or B?arrow_forwardWhat structural relationship is indicated by term D-sugar? Why are +glucose and -fructose classified as D-sugarsarrow_forward
- Sketch the titration curve for the amino acid aspartic acid.arrow_forwardIf guanine, found in the first base triplet, is removed, explain how this would affect the structure of the protein.arrow_forwardLactose permease, a protein of E. coli, is composed of a singlepolypeptide that is 417 amino acids in length. By convention, theamino acids within a polypeptide are numbered from the aminoterminalend to the carboxyl-terminal end. Are the following questionsabout lactose permease true or false?A. Because the sixty-fourth amino acid is glycine and the sixty- eighth amino acid is aspartic acid, the codon for glycine,64, is closer to the 3′ end of the mRNA than the codon for aspartic acid, 68.B. The mRNA that encodes lactose permease must be greater than1241 nucleotides in length.arrow_forward
- Indicate all the possible sequences for a tripeptide containing alanine, glutamine, and methionine; use the standard abbreviation for each amino acid (Ala, Gln, Met).arrow_forwardCysteine and Aspartate: draw the amino acid side chain and give the single and three letter abbreviation.arrow_forwardFollowing are structural formulas for cytosine and thymine. Draw two additional tautomeric forms for cytosine and three additional tautomeric forms for thymine.arrow_forward
- What is the pI, and how is it determined for amino acids that have nonionizable R groups?arrow_forwardWhen the amino acid sequences of insulin isolated from differentorganisms were determined, some differences were noted.For example, alanine was substituted for threonine, serine wassubstituted for glycine, and valine was substituted for isoleucineat corresponding positions in the protein. List the single-basechanges that could occur in triplets to produce these amino acidchanges.arrow_forwardWrite the chemical structure of peptide containing the following amino acid PRO-SER-GLY-LEUarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON
Biomolecules - Protein - Amino acids; Author: Tutorials Point (India) Ltd.;https://www.youtube.com/watch?v=ySNVPDHJ0ek;License: Standard YouTube License, CC-BY