
Concept explainers
One Lewis structure for the 2-butenyl cation is
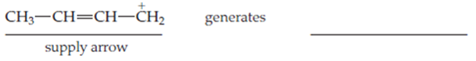
Interpretation:
The resonance structure of the given molecule is to be determined
Concept Introduction:
When the electron distribution of a molecule can be depicted correctly in more than one way using different Lewis structures, the drawings are called resonance structures.
Resonance structures have no discrete existence of their own but, taken in combination, describe the true structure of the molecule.
Answer to Problem 1EQ
The resonance structure of the given molecule is,
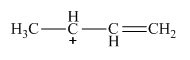
Explanation of Solution
Lewis structure for 2-butenyl cation:
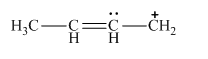
A new resonance structure can be shown as,
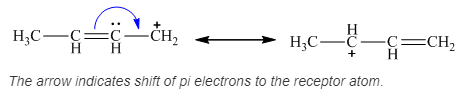
The arrow mark represents the shift of pi-electrons.
The resonance structure of the given molecule is drawn.
Want to see more full solutions like this?
Chapter 2 Solutions
Pushing Electrons
- Three resonance forms can be drawn for the molecule N2O. Which resonance form is likely to more closely resemble the structure of this molecule? (a) (b) (c)arrow_forwardNitrosyl azide, N4O, is a pale yellow solid first synthesized in 1993. Write the Lewis structure for nitrosyl azide.arrow_forwardUsing the bond dissociation enthalpies in Table 8.8, estimate the enthalpy of combustion of gaseous methane, CH4, to give water vapor and carbon dioxide gas.arrow_forward
- Write all resonance structures of chlorobenzene, C6H5Cl, a molecule with the same cyclic structure as benzene. In all structures, keep the CCl bond as a single bond. Which resonance structures are the most important?arrow_forwardSeveral Lewis structures can be written for perbromate ion, , the central Br with all single Br—O bonds, or with one, two, or three Br=O double bonds. Draw the Lewis structures of these possible resonance structures, and use formal charges to predict which makes the greatest contribution to the resonance hybrid.arrow_forwardDraw resonance formulas of the phosphoric acid molecule, (HO)3PO. Obtain formal charges for the atoms in these resonance formulas. From this result, which resonance formula would you expect to most closely approximate the actual electron distribution?arrow_forward
- does COF₂ have resonance structures?arrow_forwardConsider hypothetical elements X2 and Y2. Suppose the enthalpy of formation of the compound XY is – 84 kJ/mol, the bond energy for X2 is 105 kJ/mol, and the bond energy for Y2 is 58 kJ/mol. A)Estimate the XY bond energy, Ed, in units of kJ/mol. B)If the dissociation of a X-Y molecule were accomplished by the absorption of a single photon whose energy was exactly the quantity required, what would be its wavelength in nm? C)Would a green-light photon (λgreen=550 nm) be able to break the above X-Y bond? Explain.arrow_forwardWhat is the resonance form that describes the distribution of electrons in SeO2 ?arrow_forward
- Below are two different Lewis structures for nitrous acid (HNO2). Which is the better Lewis structure based only on formal charge?arrow_forwardAcetyl chloride, CH₃C(O)Cl, is used as a reagent for the acylation of salicylic acid in the synthesis of aspirin. Draw the Lewis structure of CH₃C(O)Cl (with minimized formal charges) and then determine if the molecule is polar or nonpolar. +arrow_forwardWhat is the formal charge of every atom in Cl2?arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning




