
For each IUPAC name, draw the corresponding structural formula and line-angle formula.
- (a) Ethanol
- (b) Butanal
- (c) Butanoic acid
- (d) Ethanoic acid
- (e) Heptanoic acid
- (f) Propanoic acid
- (g) Octanal
- (h) Cyclopentene
- (i) Cyclopentanol
- (j) Cyclopentanone
- (k) Cyclohexanol
- (l) Propanone
(a)
Interpretation:
For the given IUPAC name, the corresponding structural formula and line–angle formula has to be drawn.
Concept Introduction:
Condensed structural formula:
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Line–angle formula:
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula. This is a shorthand representation of an organic molecule with lines which represents its molecular bonding. In line–angle formula, hydrogen atoms are not shown.
Explanation of Solution
Given data:
Ethanol
Structural formula and line–angle formula:
From the name it is known that the compound has two carbon atoms. The name ends with suffix –ol which indicates that there will be an alcoholic
The structural formula and line–angle formula for ethanol is drawn below.

(b)
Interpretation:
For the given IUPAC name, the corresponding structural formula and line–angle formula has to be drawn.
Concept Introduction:
Condensed structural formula:
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Line–angle formula:
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula. This is a shorthand representation of an organic molecule with lines which represents its molecular bonding. In line–angle formula, hydrogen atoms are not shown.
Explanation of Solution
Given data:
Butanal
Structural formula and line–angle formula:
From the name it is known that the compound has four carbon atoms. The name ends with suffix –al which indicates that there will be an aldehyde
The structural formula and line–angle formula for Butanal is drawn below.
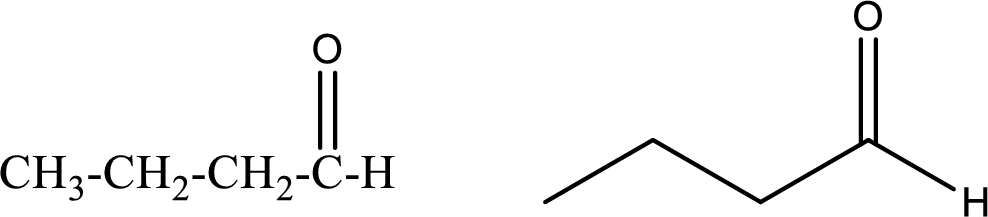
(c)
Interpretation:
For the given IUPAC name, the corresponding structural formula and line–angle formula has to be drawn.
Concept Introduction:
Condensed structural formula:
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Line–angle formula:
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula. This is a shorthand representation of an organic molecule with lines which represents its molecular bonding. In line–angle formula, hydrogen atoms are not shown.
Explanation of Solution
Given data:
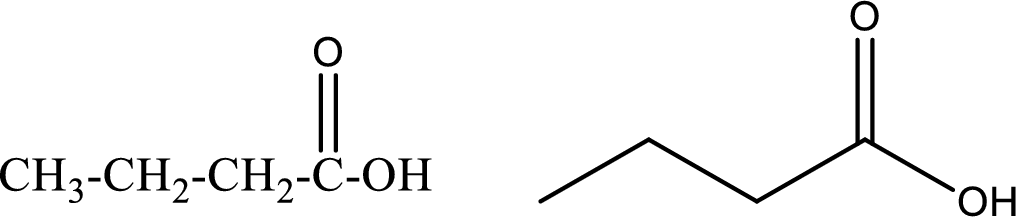
Butanoic acid
Structural formula and line–angle formula:
From the name it is known that the compound has four carbon atoms. The name ends with suffix –oic acid which indicates that there will be an acid
The structural formula and line–angle formula for butanoic acid is drawn below.
(d)
Interpretation:
For the given IUPAC name, the corresponding structural formula and line–angle formula has to be drawn.
Concept Introduction:
Condensed structural formula:
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Line–angle formula:
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula. This is a shorthand representation of an organic molecule with lines which represents its molecular bonding. In line–angle formula, hydrogen atoms are not shown.
Explanation of Solution
Given data:
Ethanoic acid
Structural formula and line–angle formula:
From the name it is known that the compound has two carbon atoms. The name ends with suffix –oic acid which indicates that there will be an acid
The structural formula and line–angle formula for ethanoic acid is drawn below.
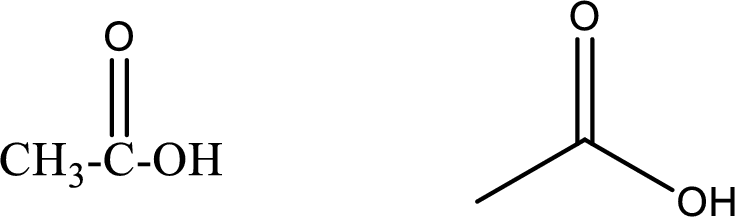
(e)
Interpretation:
For the given IUPAC name, the corresponding structural formula and line–angle formula has to be drawn.
Concept Introduction:
Condensed structural formula:
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Line–angle formula:
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula. This is a shorthand representation of an organic molecule with lines which represents its molecular bonding. In line–angle formula, hydrogen atoms are not shown.
Explanation of Solution
Given data:
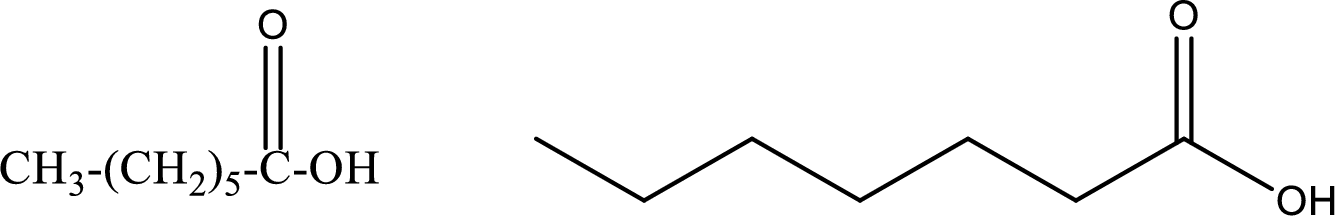
Heptanoic acid
Structural formula and line–angle formula:
From the name it is known that the compound has seven carbon atoms. The name ends with suffix –oic acid which indicates that there will be an acid
The structural formula and line–angle formula for Heptanoic acid is drawn below.
(f)
Interpretation:
For the given IUPAC name, the corresponding structural formula and line–angle formula has to be drawn.
Concept Introduction:
Condensed structural formula:
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Line–angle formula:
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula. This is a shorthand representation of an organic molecule with lines which represents its molecular bonding. In line–angle formula, hydrogen atoms are not shown.
Explanation of Solution
Given data:
Propanoic acid
Structural formula and line–angle formula:
From the name it is known that the longest chain has three carbon atoms. The name ends with suffix –oic acid which indicates that there will be an acid
The structural formula and line–angle formula for Propanoic acid is drawn below.
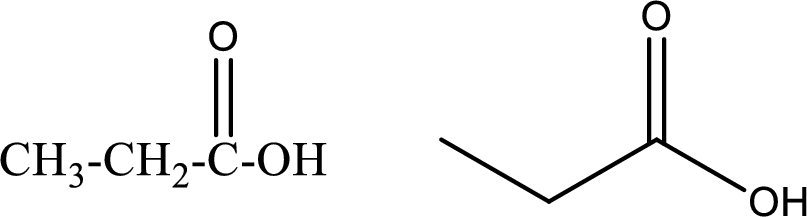
(g)
Interpretation:
For the given IUPAC name, the corresponding structural formula and line–angle formula has to be drawn.
Concept Introduction:
Condensed structural formula:
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Line–angle formula:
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula. This is a shorthand representation of an organic molecule with lines which represents its molecular bonding. In line–angle formula, hydrogen atoms are not shown.
Explanation of Solution
Given data:
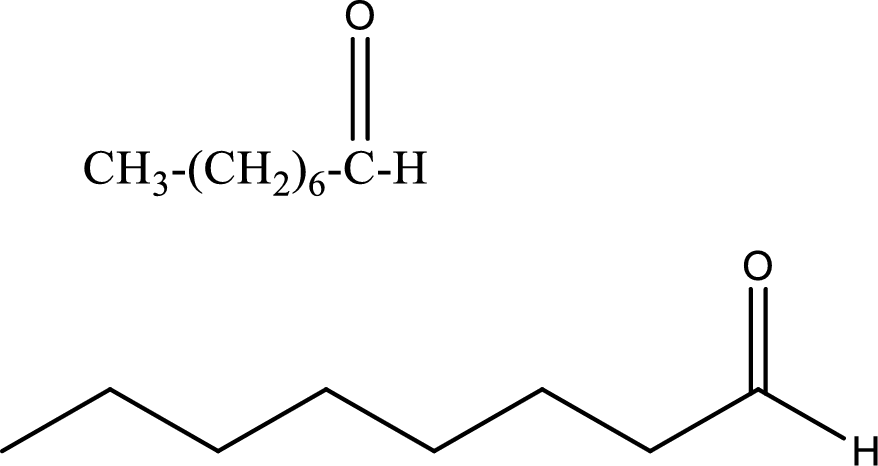
Octanal
Structural formula and line–angle formula:
From the name it is known that the longest chain has eight carbon atoms. The name ends with suffix –al which indicates that there will be an aldehyde group in the compound.
The structural formula and line–angle formula for Octanal is drawn below.
(h)
Interpretation:
For the given IUPAC name, the corresponding structural formula and line–angle formula has to be drawn.
Concept Introduction:
Condensed structural formula:
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Line–angle formula:
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula. This is a shorthand representation of an organic molecule with lines which represents its molecular bonding. In line–angle formula, hydrogen atoms are not shown.
Explanation of Solution
Given data:
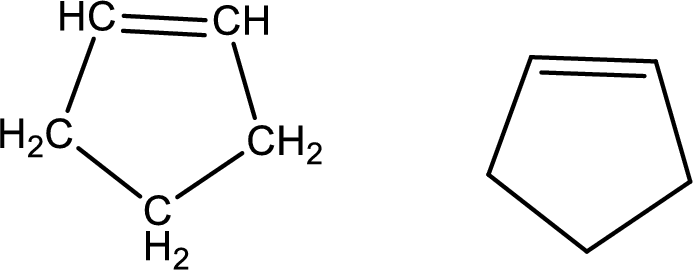
Cyclopentene
Structural formula and line–angle formula:
From the name it is known main core of the compound has a five membered cyclic ring. The name ends with suffix –ene which indicates that there will a double in the ring structure.
The structural formula and line–angle formula for Cyclopentene is drawn below.
(i)
Interpretation:
For the given IUPAC name, the corresponding structural formula and line–angle formula has to be drawn.
Concept Introduction:
Condensed structural formula:
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Line–angle formula:
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula. This is a shorthand representation of an organic molecule with lines which represents its molecular bonding. In line–angle formula, hydrogen atoms are not shown.
Explanation of Solution
Given data:
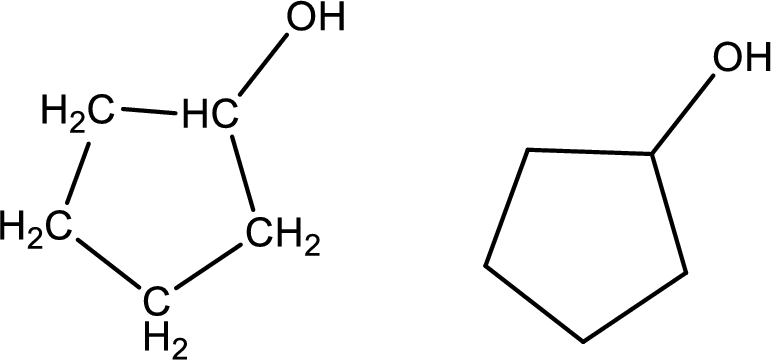
Cyclopentanol
Structural formula and line–angle formula:
From the name it is known that the main core of the compound has a five membered cyclic ring. The name ends with suffix –ol which indicates that there will be an alcoholic
The structural formula and line–angle formula for Cyclopentanol is drawn below.
(j)
Interpretation:
For the given IUPAC name, the corresponding structural formula and line–angle formula has to be drawn.
Concept Introduction:
Condensed structural formula:
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Line–angle formula:
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula. This is a shorthand representation of an organic molecule with lines which represents its molecular bonding. In line–angle formula, hydrogen atoms are not shown.
Explanation of Solution
Given data:
Cyclopentanone
Structural formula and line–angle formula:
From the name it is known that the main core of the compound has five membered cyclic ring structure. The name ends with suffix –one which indicates that there will be a ketone
The structural formula and line–angle formula for Cyclopentanone is drawn below.
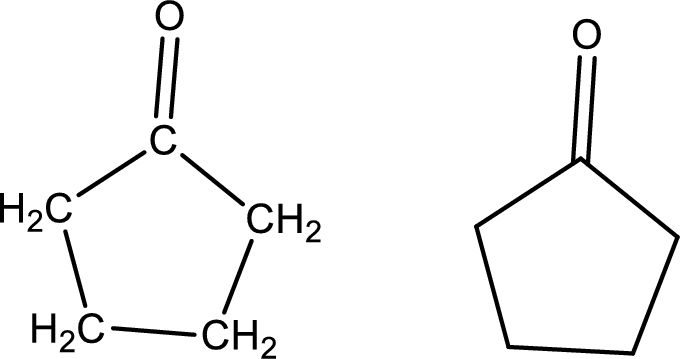
(k)
Interpretation:
For the given IUPAC name, the corresponding structural formula and line–angle formula has to be drawn.
Concept Introduction:
Condensed structural formula:
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Line–angle formula:
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula. This is a shorthand representation of an organic molecule with lines which represents its molecular bonding. In line–angle formula, hydrogen atoms are not shown.
Explanation of Solution
Given data:
Cyclohexanol
Structural formula and line–angle formula:
From the name it is known that the main core of the compound has six membered cyclic ring structure. The name ends with suffix –ol which indicates that there will be an alcoholic
The structural formula and line–angle formula for Cyclohexanol is drawn below.
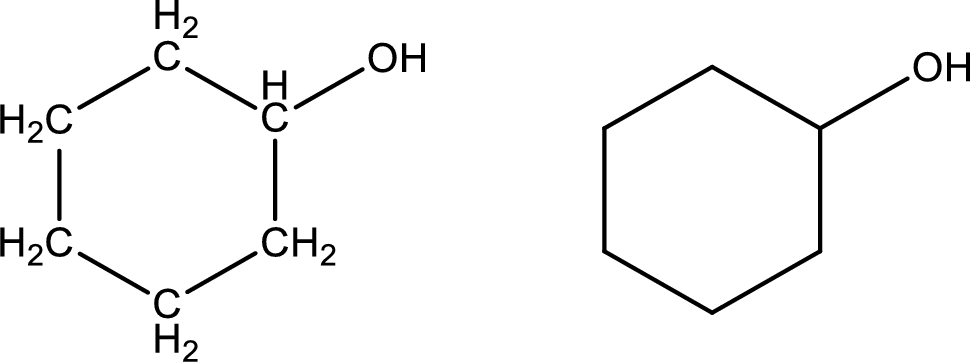
(l)
Interpretation:
For the given IUPAC name, the corresponding structural formula and line–angle formula has to be drawn.
Concept Introduction:
Condensed structural formula:
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Line–angle formula:
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula. This is a shorthand representation of an organic molecule with lines which represents its molecular bonding. In line–angle formula, hydrogen atoms are not shown.
Explanation of Solution
Given data:
Propanone
Structural formula and line–angle formula:
From the name it is known that the longest chain has three carbon atoms. The name ends with suffix –one which indicates that there will be a ketone
The structural formula and line–angle formula for Propanone is drawn below.
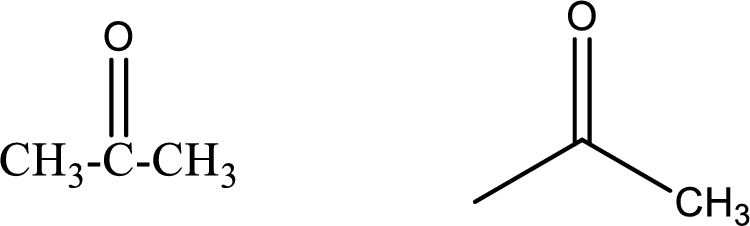
Want to see more full solutions like this?
Chapter 2 Solutions
Organic Chemistry
- Compound A and compound B are in equilibrium. Write a stepwise mechanism from compound Ato compound B showing ALL intermediates. Use curved arrows to symbolize the flow of electrons to show how each of the intermediates and products are formed. Show all lone pairs and formal charges. Lastly, explain which compound (Aor B) will be in higher concentration.arrow_forwardWhat is going on from compound B to compound C? Can you please select all the options that applyarrow_forwardAnswer the following Compounds CORRECTLY by giving the IUPAC NAME OF IT. •Label with proper notations (like hyphen, commas, periods, and grouping symbols) thank u❤️arrow_forward
- a. Which compound would you expect to have a greater dipole moment, methyl acetate or butanone?b. Which would you expect to have a higher boiling point?arrow_forwardStarting with acetylene and ethylene oxide as the only sources of carbon atoms, show how to prepare the compound Q.)Hexanedialarrow_forwardA compound C4H11N is known from its reactivity andspectroscopic properties to have no hydrogen atomsattached directly to the nitrogen atom. Write all structuralformulas consistent with this informationarrow_forward
- Please advise if these models resemble the cis-2-bromocyclohexanol & trans-2-bromocyclohexanol. Can you please advise on the priority numbers of each substituent. I am confused from the reading. Is the proproty a single element, the group of elements, the left and right carbon atoms? Please advise and give commentary on Br. & Methyl group oriantation related to the doubl carbon bond plain. And if you can provide a visual that would be even better for the futre, the more detail the better my study notes will be. Thank you. Justinarrow_forwardgive the structural formula for compounds A to Garrow_forwardA chemist isolated an aromatic compound with molecular formula C6H4Br2. He treated this compound with nitric acid and sulfuric acid and isolated threedifferent isomers, in different amounts, with molecular formula C6H3Br2NO2. What was the structure of the original compound?arrow_forward
- what is the milliequivalent weight of acetic acid?arrow_forwardWhich of the following statements is INCORRECT concerning the two acids below? Options: Acetic acid has higher water solubility than benzoic acid. Acetic acid has a higher melting point than benzoic acid. Benzoic acid has a larger conjugated system. Benzoic acid is the stronger acid. Acetic acid has an electron donating group in it's structure.arrow_forwardProvide a detailed, stepwise mechanism for the following transformation. Use the curved arrow formalism to show the flow of electrons. Show all lone pairs, formal charges, etcarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
