
Concept explainers
Which compounds can be deprotonated by
(a).
(b).
(c). 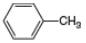
(d).
Interpretation: Among the given compounds, those which are deprotonated by
Concept Introduction: According to Bronsted-Lowry theory, when an acid donates a proton the species formed is known as conjugate base and when the base accepts a proton the species formed is known as conjugate acid.
In a reaction which strongly favors the formation of products, the base used to remove proton from the acid should be stronger than the base formed when the proton is removed. In a reaction which favors the products, equilibrium will favors the formation of the weaker acid or weaker base. Or the
Answer to Problem 2.48P
Correct answer: The correct options are (a) and (b).
Explanation of Solution
Reason for correct answer:
(a) The deprotonation of an acid takes place in the presence of a strong base whose
The given compound is formic acid,
The
(b) The given compound is hydrogen sulphide,
The
Reason for incorrect options:
(c) The given compound is toluene,
The
(d) The given compound is methyl amine,
The
Among the given compounds, formic acid,
Want to see more full solutions like this?
Chapter 2 Solutions
Organic Chemistry
- Based on the pH measured for the solution of (NH4)2CO3, is NH4+ is a stronger acid or is CO2−3 stronger base? and Why?arrow_forwardPredict the products of the following acid-base reactions, and predict whether the equilibrium lies to the left or to the right of the reaction arrow. HSO4−(aq)+CO3 2−(aq)⇌arrow_forwardCalculate the pKa value for each of the following acids. a. Nitrous acid (HNO2), Ka = 4.5 104 b. Carbonic acid (H2CO3), Ka = 4.3 107 c. Dihydrogen phosphate ion (H2PO4), Ka = 6.2 108 d. Sulfurous acid (H2SO3), Ka = 1.5 102arrow_forward
- What are the major species in solution after NaHSO4 is dissolved in water? What happens to the pH of the solution as more NaHSO4 is added? Why? Would the results vary if baking soda (NaHCO3) were used instead?arrow_forwardComplete the following equations. If no reaction occurs, write no reaction. a. b. c. d. e.arrow_forwardWhat are the major species present in 0.250 M solutions of each of the following acids? Calculate the pH of each of these solutions. a. HClO4 b. HNO3arrow_forward
- Apatite, Ca5(PO4)3OH, is the mineral in teeth. On a chemical basis explain why drinking milk strengthens young children’s teeth. Sour milk contains lactic acid. Not removing sour milk from the teeth of young children can lead to tooth decay. Use chemical principles to explain why.arrow_forwardDuring respiration, lungs expel carbon dioxide, leading to the formation of more carbonic acid (H2CO3), which then dissociates to replenish the loss of carbon dioxide. This phenomenon is an example of a) Acid-Base catalysis b) Le Chatelier's Principle c) Buffering capacity d) Henderson Hasselbalch Relationshiparrow_forwardCalculate the equilibrium constant for the acid–base reaction between the reactants in each of the following pairs: (a) HCl + H2O (b) CH3COOH + H2O (c) CH3NH2 + H2O (d) CH3N+H3 + H2Oarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





