
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 2.62P
Following is a planar hexagon representation of L-fucose, a sugar component of the determinants of the A, B, O blood group typing. For more on this system of blood typing, see “Chemical Connections: A, B, AB, and O Blood Group Substances” in Chapter 25.
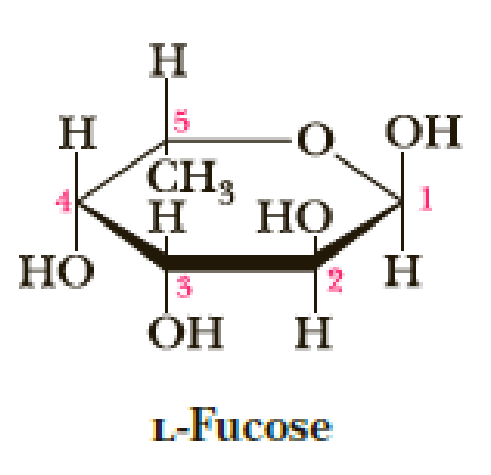
- (a) Draw the alternative chair conformations of L-fucose.
- (b) Which of them is more stable? Explain.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
The following are representations of two forms of glucose. The six-membered ring is known to exist in a chair conformation in each form. Draw clear representations of the most stable conformation of each. Are they two different conformations of the same molecule, or are they stereoisomers that cannot be interconverted by rotation about single bonds? Which substituents (if any) occupy axial sites?
Identify the stereogenic carbon in (S)- and (R)-limonene, rank the substituents around it and rationalize the assignment of their stereochemical configurations. Hint: When ranking carbons that have multiple bonds, consider the bolded carbon of C=C being connected to 2 carbons and the bolded carbon of C≡C being connected to 3 carbons.
Which do you expect to be the more stable conformation of cis-1,3-dimethylcyclobutane, A or B? Why?
Chapter 2 Solutions
Organic Chemistry
Ch. 2.2 - Do the line-angle formulas in each pair represent...Ch. 2.2 - Draw line-angle formulas for the three...Ch. 2.3 - Write IUPAC names for these alkanes.Ch. 2.4 - Combine the proper prefix, infix, and suffix and...Ch. 2.4 - Write the molecular formula, IUPAC name, and...Ch. 2.4 - Write molecular formulas for each bicycloalkane,...Ch. 2.4 - Prob. 2.7PCh. 2.5 - For 1,2-dichloroethane: (a) Draw Newman...Ch. 2.5 - Following is a chair conformation of cyclohexane...Ch. 2.5 - Draw the alternative chair conformation for the...
Ch. 2.5 - Draw a chair conformation of...Ch. 2.6 - Which cycloalkanes show cis, trans isomerism? For...Ch. 2.6 - Following is a planar hexagon representation for...Ch. 2.6 - Here is one cis,trans isomer of...Ch. 2.6 - Prob. AQCh. 2.6 - Prob. BQCh. 2.6 - Prob. CQCh. 2.7 - Arrange the alkanes in each set in order of...Ch. 2 - Write a line-angle formula for each condensed...Ch. 2 - Write the molecular formula of each alkane.Ch. 2 - Using parentheses and subscripts, provide an even...Ch. 2 - Which statements are true about constitutional...Ch. 2 - Prob. 2.20PCh. 2 - Each member of the following set of compounds is...Ch. 2 - Each of the following compounds is an amine...Ch. 2 - Each of the following compounds is either an...Ch. 2 - Draw structural formulas and write IUPAC names for...Ch. 2 - Draw structural formulas for all of the following....Ch. 2 - Write IUPAC names for these alkanes and...Ch. 2 - Write structural formulas and line-angle formulas...Ch. 2 - Explain why each is an incorrect IUPAC name and...Ch. 2 - For each IUPAC name, draw the corresponding...Ch. 2 - Write the IUPAC name for each compound.Ch. 2 - Prob. 2.31PCh. 2 - Torsional strain resulting from eclipsed CH bonds...Ch. 2 - How many different staggered conformations are...Ch. 2 - Consider 1-bromopropane, CH3CH2CH2Br. (a) Draw a...Ch. 2 - Consider 1-bromo-2-methylpropane and draw the...Ch. 2 - trans-1,4-Di-tert-butylcyclohexane exists in a...Ch. 2 - From studies of the dipole moment of...Ch. 2 - Prob. 2.38PCh. 2 - Following are the alternative chair conformations...Ch. 2 - Prob. 2.40PCh. 2 - Prob. 2.41PCh. 2 - Draw line-angle formulas for the cis and trans...Ch. 2 - Name and draw structural formulas for all...Ch. 2 - Using a planar pentagon representation for the...Ch. 2 - Gibbs free energy differences between...Ch. 2 - Prob. 2.46PCh. 2 - Calculate the difference in Gibbs free energy in...Ch. 2 - Draw the alternative chair conformations for the...Ch. 2 - Use your answers from Problem 2.48 to complete the...Ch. 2 - There are four cis,trans isomers of...Ch. 2 - Draw alternative chair conformations for each...Ch. 2 - 1,2,3,4,5,6-Hexachlorocyclohexane shows cis,trans...Ch. 2 - Prob. 2.53PCh. 2 - What generalization can you make about the...Ch. 2 - What unbranched alkane has about the same boiling...Ch. 2 - Complete and balance the following combustion...Ch. 2 - Following are heats of combustion per mole for...Ch. 2 - Following are structural formulas and heats of...Ch. 2 - Without consulting tables, arrange these compounds...Ch. 2 - Which would you predict to have the larger (more...Ch. 2 - Following are structural formulas for 1,4-dioxane...Ch. 2 - Following is a planar hexagon representation of...Ch. 2 - On the left is a stereorepresentation of glucose...Ch. 2 - Prob. 2.64PCh. 2 - Prob. 2.65P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider 1-bromo-2-methylpropane and draw the following. (a) The staggered conformation(s) of lowest energy (b) The staggered conformation(s) of highest energyarrow_forwardKetones react with alcohols to yield products called acetals. Why does the all-cis isomer of 4-tert-butyl-1,3-cyclohexanediol react readily with acetone and an acid catalyst to form an acetal, but other stereoisomers do not react? In formulating your answer, draw the more stable chair conformations of all four stereoisomers and the product acetal for each one.arrow_forwardGiven that the free energy of the twist-boat conformer of cyclohexane is 5.3 kcal/mol greater than that of the chair conformer, calculate the percentage of twist-boat conformers present in a sample of cyclohexane at 25 °C. Does your answer agree with the statement made in Section 3.13 about the relative number of molecules in these two conformations?arrow_forward
- Draw the two chair conformations of menthol, and identify the more stable conformation. Explain brieflyarrow_forwardProvide a Newman projection of a staggered conformation along the C2-C3 bond of the structure below.arrow_forwardConsider 1-bromopropane, CH3CH2CH2Br. (a) Which of these is the lowest energy conformation?arrow_forward
- Draw 1-methyl-3-isopropylcyclohexane in its most stable conformation. Hint: Please note that the most stable conformation of cychohexane is NOT a planar hexagonarrow_forwardwhat is the sawhorse projection of the LEAST STABLE ECLIPSED CONFORMATION OF N-PENTANE ROTATING BETWEEN C2-C3?arrow_forwardWhat is the highest energy conformation of 3-methylpentane when viewed down the 2-3 carbon-carbon bond?arrow_forward
- Define the degree of unsaturation ?arrow_forwardIdentify the equilibrium below that shows the two chair conformations of the following compound: Please don't provide handwriting solutionarrow_forwardDraw each of the above molecules in a 3D perspective. Show all six-membered rings as chairs and all acyclic torsions in staggered conformation. If multiple conformations are possible, choose the best one.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License