
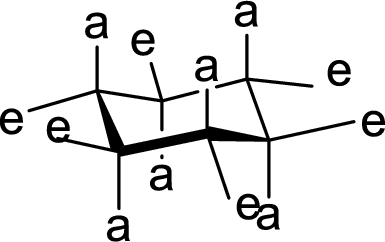
Concept explainers
(a)
Interpretation:
The conformation of rings A, B, C and D in Cholestanol has to be described.
Concept Introduction:
The conformation structures of six membered rings are given by chair forms. In the chair conformation, all
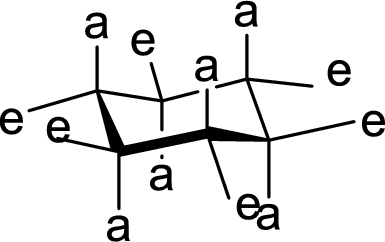
(b)
Interpretation:
The hydroxyl group present on Ring A is either axial or equatorial has to be given.
Concept Introduction:
The
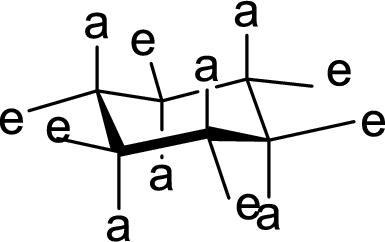
(c)
Interpretation:
The methyl group present at the junction of Ring A and Ring B is either axial or equatorial to Ring A and Ring B has to be given.
Concept Introduction:
The
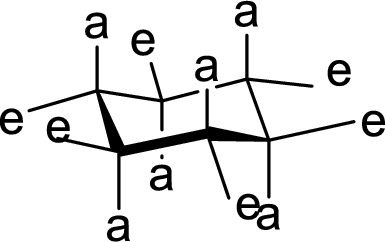
(d)
Interpretation:
The methyl group present at the junction of Ring C and Ring D is either axial or equatorial has to be given.
Concept Introduction:
The
Trending nowThis is a popular solution!

Chapter 2 Solutions
Organic Chemistry
- a) When (Z)-3-methylhex-3-ene undergoes hydroboration–oxidation, two isomeric products are formed. Give their structures, and label each asymmetric carbon atom as (R) or (S). What is the relationship between these isomers?arrow_forwardFive isomeric alkanes (A–E) having the molecular formula C6H14 are each treated with Cl2 + hv to give alkyl halides having molecular formulaC6H13Cl. A yields five constitutional isomers. B yields four constitutionalisomers. C yields two constitutional isomers. D yields threeconstitutional isomers, two of which possess stereogenic centers. Eyields three constitutional isomers, only one of which possesses astereogenic center. Identify the structures of A–E.arrow_forwardCompound X and compound Y are constitutional isomers with the molecular formula C5H10. Compound X possesses a carbon-carbon double bond in the trans configuration, while compound Y possesses a carbon-carbon double bond that is not stereoisomeric:arrow_forward
- Butylated hydroxytoluene (BHT) is a common preservativeadded to cereals and other dry foods. Its systematic name is 1-hydroxy-2,6-di-tert-butyl-4-methylbenzene (where “tert-butyl”is 1,1-dimethylethyl). Draw the structure of BHT.arrow_forwardBiphenyl has the following structure.(a) Is biphenyl a (fused) polynuclear aromatic hydrocarbon?(b) How many pi electrons are there in the two aromatic rings of biphenyl? How does this number compare with that for naphthalene?arrow_forwardMaltose is a carbohydrate present in malt, the liquid obtained from barleyand other grains. Although maltose has numerous functional groups, itsreactions are explained by the same principles we have alreadyencountered. Label the acetal and hemiacetal carbons.arrow_forward
- Are the hydroxyl groups on rings A, B, and C axial or equatorial to their respective rings?arrow_forwardThe following are representations of two forms of glucose. The six-membered ring is known to exist in a chair conformation in each form. Draw clear representations of the most stable conformation of each. Are they two different conformations of the same molecule, or are they stereoisomers that cannot be interconverted by rotation about single bonds? Which substituents (if any) occupy axial sites?arrow_forwardWhich of the isomeric alcohols having the molecular formula C6H14O are chiral? Which are achiral?arrow_forward
- Two constitutional isomers with molecular formula C2H6O have boiling points of –23.6°C and 78.4°C. Draw both constitutional isomers of C2H6O (or provide the name). Identify the structural class of each. Match the constitutional isomers you have drawn to the boiling points provided above, and explain your reasoning.arrow_forwardAlcohol A (C10H18O) is converted to a mixture of alkenes B and C on being heated with potassium hydrogen sulfate (KHSO4). Catalytic hydrogenation of B and C yields the same product. Assuming that dehydration of alcohol A proceeds without rearrangement, deduce the structures of alcohol A and alkene C.arrow_forwardAn unknown hydrocarbon A with the formula C6H12 reacts with 1 molar equivalent of H2 over a palladium catalyst. Hydrocarbon A also reacts with OsO4 to give diol B. When oxidized with KMnO4 in acidic solution, A gives two fragments. One fragment is propanoic acid, CH3CH2CO2H, and the other fragment is ketone C. What are the structures of A, B, and C? Write all reactions, and show your reasoning.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
