
Concept explainers
(a)
Interpretation:
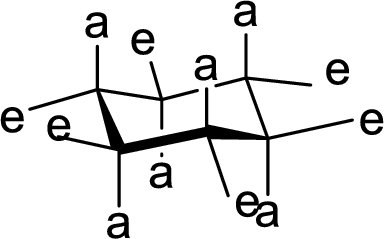
The conformation of rings A, B, C and D in Cholic acid has to be described.
Concept Introduction:
The conformation structures of six membered rings are given by chair forms. In the chair conformation, all
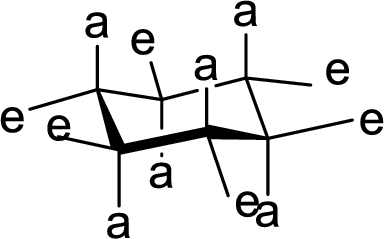
(b)
Interpretation:
The hydroxyl group present on Ring A, B and C are either axial or equatorial to their respective rings has to be given.
Concept Introduction:
The
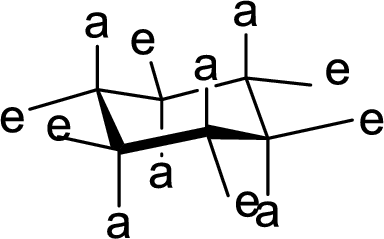
(c)
Interpretation:
The methyl group present at the junction of Ring A and Ring B is either axial or equatorial to Ring A and Ring B has to be given.
Concept Introduction:
The
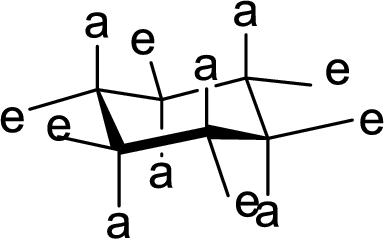
(d)
Interpretation:
The hydrogen present at the junction of Ring A and Ring B is either axial or equatorial to Ring A and Ring B has to be given.
Concept Introduction:
The
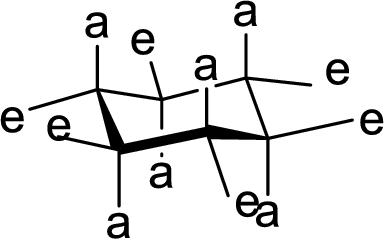
(e)
Interpretation:
The methyl group present at the junction of Ring C and Ring D is either axial or equatorial to Ring C has to be given.
Concept Introduction:
The
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
Organic Chemistry
- Following is a structural formula for cortisol (hydrocortisone). Draw a stereo-representation of this molecule showing the conformations of the five- and six-membered rings.arrow_forward(a) Draw a skeletal structure of the anabolic steroid methenolone from the following description. Methenolone contains the tetracyclic steroid skeleton with a carbonyl group at C3, a hydroxyl at C17, a double bond between C1 and C2, and methyl groups bonded to C1, C10, and C13. (b) Add wedges and dashed wedges for all stereogenic centers with thefollowing information: the configuration at C10 is R, the configuration at C13 is S, the configuration at C17 is S, and all substituents at ring fusions are trans to each other. (c) Draw the structure of Primobolan, the product formed when methenolone is treated with CH3(CH2)5COCl and pyridine. Primobolan is an anabolic steroid that can be taken orally or by injection and has been used illegally by well-known Major League Baseball players.arrow_forwardWrite structural formulas for all ketones with the molecular formula C6H12O and give each its IUPAC name. Which of these ketones are chiral?arrow_forward
- Read Appendix B on naming branched alkyl substituents, and draw all possible alkyl groups having the formula C5H11–. Give the IUPAC names for the eight compounds of molecular formula C10H20 that contain a cyclopentane ring with each of these alkyl groups as a substituent.arrow_forwarda. What alkane, with molecular formula C5H12, forms only one monochlorinated product when it is heated with Cl2? b. What alkane, with molecular formula C7H16, forms seven monochlorinated products (disregarding stereoisomers) when heated with Cl2?arrow_forwardAre the hydroxyl groups on rings A, B, and C axial or equatorial to their respective rings?arrow_forward
- Consider the substituted cyclohexane shown in the ball-and-stick model. (See attached) Are the substituents on C2 and C4 cis or trans to each other?arrow_forwardWrite a conformational structure for 1,2,3-trimethylcyclohexane in which all the methyl groups are axial and then show its more stable conformation.arrow_forwardWhy is a substituted cyclohexane ring more stable with a larger group in the equatorial position?arrow_forward
- Draw and name the six isomeric cyclopentane of molecular formula C7H14. These will include four constitutional isomers, of which two show geometric (cis-trans) stereoisomerism.arrow_forwardDraw all the isomers with molecular formula C6H12 that contain a cyclobutane ring. (Hint: There are seven.)arrow_forwardCompounds A and B are isomers of the molecular formula C9H19Br. Both yield the same alkene C in an elimination reaction. Hydrogenation of C yields the product 2,3,3,4 tetramethyl pentane. What are the structures of A, B, and C?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
