
Concept explainers
What is the hybridization of each carbon in
Interpretation:
The hybridization of each carbon atom in the given molecule is to be determined along with the
Concept introduction:
A carbon atom is said to be
Multiple bonds are treated as a unit while determining the hybridization of different atoms in a molecule. For
Answer to Problem 26P
Solution:
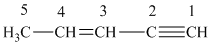
Carbon
Carbon
Carbon
Carbon
Carbon
For carbon
For carbon
For carbon
Explanation of Solution
The structure for the given compound is:
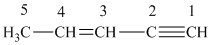
We number each carbon atom in the above compound.
A carbon atom is said to be
A carbon atom is said to be
A carbon atom is said to be
For carbon
For carbon
For carbon
The hybridization of each carbon atom in the given molecule is
Want to see more full solutions like this?
Chapter 2 Solutions
Organic Chemistry - Standalone book
- Gamma hydroxybutyric acid, GHB, infamous as a date rape drug, is used illicitly because of its effects on the nervous system. The condensed molecular formula for GHB is HO(CH2)3COOH. (a) Write the Lewis structure for GHB. (b) Identify the hybridization of the carbon atom in the CH2 groups and of the terminal carbon. (c) Is hydrogen bonding possible in GHB? If so, write Lewis structures to illustrate the hydrogen bonding. (d) Which carbon atoms are involved in sigma bonds? In pi bonds? (e) Which oxygen atom is involved in sigma bonds? In pi bonds?arrow_forwardAspirin, or acetylsalicylic acid, has the formula C9H8O4 and the skeleton structure (a) Complete the Lewis structure and give the number of bonds and bonds in aspirin. (b) What is the hybridization about the CO2H carbon atom (colored blue)? (c) What is the hybridization about the carbon atom in the benzene-like ring that is bonded to an oxygen atom (colored red)? Also, what is the hybridization of the oxygen atom bonded to this carbon atom?arrow_forwardThere are two compounds with the molecular formula HN3. One is called hydrogen azide; the other is cyclotriazene. (a) Write the Lewis structure for each compound. (b) Designate the hybridization of each nitrogen in hydrogen azide. (c) What is the hybridization of each nitrogen in cyclotriazene? (d) How many sigma bonds are in hydrogen azide? In cyclotriazene? (e) How many pi bonds are in hydrogen azide? In cyclotriazene? (f) Give approximate values for the N-to-N-to-N bond angles in each molecule.arrow_forward
- The hybridization of the two carbon atoms differs in an acetic acid, CH3COOH, molecule. (a) Designate the correct hybridization for each carbon atom in this molecule. (b) What is the approximate bond angle around each carbon?arrow_forwardMany important compounds in the chemical industry are derivatives of ethylene (C2H4). two of them are acrylonitrile and methyl methacrylate. Acrylonitrile Methyl methacrylate Complete the Lewis structures, showing all lone pairs. Give approximate values for bond angles a through f Give the hybridization of all carbon atoms. In acrylonitrile, how many of the atoms in the molecule must lie in the same plane? How many bonds and how many bonds are there in methyl methacrylate and acrylonitrile?arrow_forwardWhat is the hybridization of carbon in acetylene, C2H2 (HCCH)? W: unhybridized X: sp Y: sp2 Z: sp3arrow_forward
- What are the angles at atom C(5)?arrow_forwardDraw a line-bond structure for propyne, CH3Ca%!CH. Indicate the hybridization of the orbitals on each carbon, and predict a value for each bond angle.arrow_forwardWhy do all these atoms lie in the same plane? Is it because we have 2 triagnol planars and an N-C triple bond with 180 degree angles?arrow_forward
- Section 7.3 shows that the compound 2-butene exists intwo isomeric forms, which can be interconverted only bybreaking a bond (in that case, the central double bond).How many possible isomers correspond to each of the following chemical formulas? Remember that a simple rotation of an entire molecule does not give a different isomer.Each molecule contains a central CuC bond.(a) C2H2Br2(b) C2H2BrCl(c) C2HBrClFarrow_forwardWhat is the molecular structure about the nitrogen atom in trimethyl amine and in the trimethyl ammonium ion, (CH3)3NH+? What is the hybridization of the nitrogen atom in trimethyl amine and in the trimethyl ammonium ion?arrow_forwardDraw and describe the hybridization process of PROPENE, CH3CH=CH2. State the type of hybrid of each carbon, label all the bonds involve.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning


