
Chemistry for Engineering Students
4th Edition
ISBN: 9781337398909
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 2.74PAE
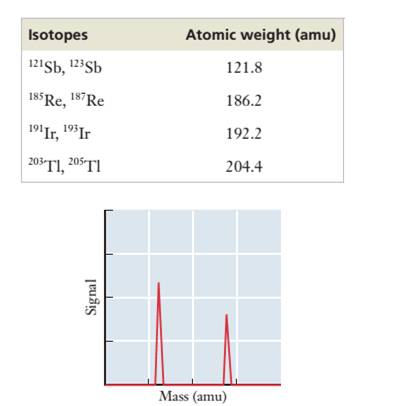 2.74 The accompanying table provides the identity of the two naturally occurring
2.74 The accompanying table provides the identity of the two naturally occurring
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 2 Solutions
Chemistry for Engineering Students
Ch. 2 - Name at least three common polymers and give...Ch. 2 - Prob. 2COCh. 2 - Describe the nuclear model for the atom and...Ch. 2 - Prob. 4COCh. 2 - Prob. 5COCh. 2 - Prob. 6COCh. 2 - Prob. 7COCh. 2 - Prob. 8COCh. 2 - Prob. 9COCh. 2 - Prob. 10CO
Ch. 2 - Prob. 2.1PAECh. 2 - How do polymers compare to their respective...Ch. 2 - Look around you and identify several objects that...Ch. 2 - Prob. 2.4PAECh. 2 - The fact that a polymer’s physical properties...Ch. 2 - One application of conductive polymers is in...Ch. 2 - Prob. 2.7PAECh. 2 - Prob. 2.8PAECh. 2 - Why is the number of protons called the atomic...Ch. 2 - 2.10 Which isotope in each pair contains more...Ch. 2 - 2.11 Define the term isotope.Ch. 2 - 2.12 Write the complete atomic symbol for each of...Ch. 2 - 2.13 How many electrons, protons, and neutrons are...Ch. 2 - 2.14 Consider the following nuclear symbols. How...Ch. 2 - 2.15 Mercury is 16.716 times more massive than...Ch. 2 - The element gallium, used in gallium arsenide...Ch. 2 - 2.17 The atomic weight of copper is 63.55 amu....Ch. 2 - The following table presents the abundances and...Ch. 2 - 2.19 Naturally occurring uranium consists of two...Ch. 2 - Prob. 2.20PAECh. 2 - Prob. 2.21PAECh. 2 - 2.22 Provide the symbol of the following...Ch. 2 - Prob. 2.23PAECh. 2 - 2.24 Identify each of the following species as an...Ch. 2 - 2.25 Write the atomic symbol for the element whose...Ch. 2 - 2.26 In what region of the periodic table are you...Ch. 2 - Prob. 2.27PAECh. 2 - Prob. 2.28PAECh. 2 - Prob. 2.29PAECh. 2 - 2.30 Using Coulomb’s law, explain how the...Ch. 2 - Prob. 2.31PAECh. 2 - 2.32 Which of the following formulas contains the...Ch. 2 - Prob. 2.33PAECh. 2 - Prob. 2.34PAECh. 2 - Prob. 2.35PAECh. 2 - 2.36 Explain the difference between a molecular...Ch. 2 - 2.37 Why are empirical formulas preferred for...Ch. 2 - 2.38 The molecular formula for the ethylene...Ch. 2 - 239 Polybutadiene is a synthetic elastomer, or...Ch. 2 - 2.40 What distinguished the work of Mendeleev that...Ch. 2 - 2.41 How does the periodic table help to make the...Ch. 2 - 2.42 What is a period in the periodic table? From...Ch. 2 - 2.43 Name of the group to which each of the...Ch. 2 - Prob. 2.44PAECh. 2 - Prob. 2.45PAECh. 2 - 2.46 Why are nonmetals important even though they...Ch. 2 - Prob. 2.47PAECh. 2 - A materials engineer has filed for a patent for a...Ch. 2 - Prob. 2.49PAECh. 2 - 2.50 A materials engineer wants to make a new...Ch. 2 - Prob. 2.51PAECh. 2 - Prob. 2.52PAECh. 2 - 2.53 What is meant by the phrase organic...Ch. 2 - 2.54 Based on what you have learned in this...Ch. 2 - 2.55 What is a functional group? How does the...Ch. 2 - Prob. 2.56PAECh. 2 - Prob. 2.57PAECh. 2 - Prob. 2.58PAECh. 2 - 2.59 The accompanying figure shows the structure...Ch. 2 - Prob. 2.60PAECh. 2 - 2.61 Name the following covalent compounds: (a)...Ch. 2 - Prob. 2.62PAECh. 2 - Prob. 2.63PAECh. 2 - Prob. 2.64PAECh. 2 - Prob. 2.65PAECh. 2 - Prob. 2.66PAECh. 2 - Prob. 2.67PAECh. 2 - 2.68 What is a free radical? How are free radicals...Ch. 2 - Prob. 2.69PAECh. 2 - 2.70 Why do you think an inhibitor molecule is...Ch. 2 - 2.71 Use the web to determine the amount of...Ch. 2 - 2.72 How can an element have an atomic weight that...Ch. 2 - 2.73 Explain the concept of a “weighted” average...Ch. 2 - 2.74 The accompanying table provides the identity...Ch. 2 - 2.75 Chlorine has only two isotopes, one with mass...Ch. 2 - Prob. 2.76PAECh. 2 - Prob. 2.77PAECh. 2 - Prob. 2.78PAECh. 2 - Prob. 2.79PAECh. 2 - 2.80 Of the following elements, which two would...Ch. 2 - 2.81 How do binary compounds with hydrogen...Ch. 2 - Prob. 2.82PAECh. 2 - Prob. 2.83PAECh. 2 - 2.84 Early attempts to arrange the elements often...Ch. 2 - 2.85 Describe how the saying “opposites attract”...Ch. 2 - 2.86 For some uses, the relative abundance of...Ch. 2 - 2.87 What is the heaviest element to have an...Ch. 2 - 2.88 Describe how you can identify the isotope, X,...Ch. 2 - Prob. 2.89PAECh. 2 - 2.90 Naturally occurring europium has an average...Ch. 2 - 2.91 Strontium has four stable isotopes....Ch. 2 - 2.92 A candy manufacturer makes chocolate-covered...Ch. 2 - Prob. 2.93PAECh. 2 - 2.94 Use a molecular level description to...Ch. 2 - 2.95 Engineers who design bicycle frames are...Ch. 2 - 2.96 Use the web to look up the density of...Ch. 2 - 2.97 LDPE has a density in the range of...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give the complete symbol(ZAX), including atomic number and mass number, of (a) a nickel atom with 31 neutrons, (b) a plutonium atom with 150 neutrons, and (c) a tungsten atom with 110 neutrons.arrow_forwardFrom the following written description, write the balanced chemical equation for the reaction including state symbols. A diatomic gaseous molecule that contains 17 protons per atom is reacted with a solid element that has an atomic number of 19 to yield an ionic compound.arrow_forward2.90 Naturally occurring europium has an average atomic weight of 151.964 amu. If the only isotopes of europium present are 151Eu and 153Eu, describe how you would determine the relative abundance of the two isotopes. Include in your description any information that would need to be looked up.arrow_forward
- Give the complete symbol (XZA), including atomic number and mass number, of (a) a nickel atom with 31 neutrons, and (b) a tungsten atom with 110 neutrons.arrow_forwardNaturally occurring nitrogen is a mixture of 14N and 15N. Give the number of protons, neutrons, and electrons in the neutral atom of each isotope.arrow_forwardIn a hypothetical universe, an oil-drop experiment gave the following measurements of charges on oil drops: 5.55 1019 C, 9.25 1019 C, 1.11 1018 C, and 1.48 1018 C. Assume that the smallest difference in charge equals the unit of negative charge in this universe. What is the value of this unit of charge? How many units of excess negative charge are there on each oil drop?arrow_forward
- Model of the Atom Consider the following depictions of two atoms, which have been greatly enlarged so you can see the subatomic particles. a How many protons are present in atom A? b What is the significance of the number of protons depicted in atom A or any atom? c Can you identify the real element represented by the drawing of atom A? If so, what element does it represent? d What is the charge on atom A? Explain how you arrived at your answer. e Write the nuclide symbol of atom A. f Write the atomic symbol and the atomic number of atom B. g What is the mass number of atom B? How does this mass number compare with that of atom A? h What is the charge on atom B? i Write the nuclide symbol of atom B. j Draw pictures like those above of 36Li+ and 36Li atoms. What are the mass number and atomic number of each of these atoms? k Consider the two atoms depicted in this problem and the two that you just drew. What is the total number of lithium isotopes depicted? How did you make your decision? l Is the mass number of an isotope of an atom equal to the mass of the isotope of the atom? Be sure to explain your answer.arrow_forwardThough the common isotope of aluminum has a mass number of 27, isotopes of aluminum have been isolated (or prepared in nuclear reactors) with mass numbers of 24, 25, 26, 28, 29, and 30. How many neutrons are present in each of these isotopes? Why are they all considered aluminum atoms, even though they differ greatly in mass? Write the atomic symbol for each isotope.arrow_forwardThe following table gives the number of protons and neutrons in the nuclei of various atoms. Which atom is the isotope of atom A? Which atom has the same mass number as atom A? Protons Neutrons Atom A 18 19 Atom B 16 19 Atom C 18 18 Atom D 17 20arrow_forward
- 2-27 If each atom in Problem 2-26 acquired two more neutrons, what element would each then be?arrow_forwardDefine mass number. What is the difference between mass number and atomic mass?arrow_forwardWhile traveling to a distant universe, you discover the hypothetical element X. You obtain a representative sample of the element and discover that it is made up of two isotopes, X-23 and X-25. To help your science team calculate the atomic weight of the substance, you send the following drawing of your sample with your report. In the report, you also inform the science team that the brown atoms are X-23, which have an isotopic mass of 23.02 amu, and the green atoms are X-25, which have an isotopic mass of 25.147 amu. What is the atomic weight of element X?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
The Bohr Model of the atom and Atomic Emission Spectra: Atomic Structure tutorial | Crash Chemistry; Author: Crash Chemistry Academy;https://www.youtube.com/watch?v=apuWi_Fbtys;License: Standard YouTube License, CC-BY