
(a)
Interpretation:
Rutherford’s experiment has to be described and how it led to the structure of the atom should be explained. Also, how was he able to estimate the number of protons in a nucleus from the scattering of alpha particles should be explained.
Concept Introduction:
Atoms: Atoms consist of tiny particles called protons, neutrons and electrons. Proton and neutrons are present in the nucleus and the electron resides around the nucleus. The protons number will be same as the electrons count in the atom.
The
Where,
(a)
Explanation of Solution
The atom consists of concentrated mass called nucleus at the centre and surrounded by the electrons.
Every atom contains a nucleus in which all of its positive charge and most of its mass are concentrated is proven by the experiment.
Experiment:
Allowing alpha-particles (positively charged) to bombard with the gold foil, expected all the rays to pass through. In contrast, the rays are deflected with angles is observed.
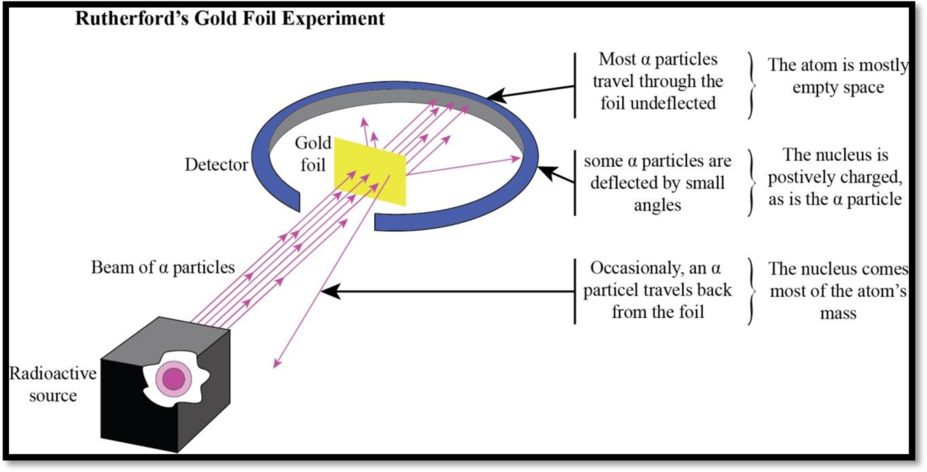
Figure 1
Rutherford’s Experiment evidences about:
- The atom mostly consist of empty space (due to empty space most of the rays went through the foil).
- Very solid particles; presence of nucleus is revealed by the rays that bounced back.
- The nucleus consisting of positive charge is confirmed by the rays deflected at an angle. The similar charges have no attraction and are deflected away.
(b)
Interpretation:
The density of the nucleus
Concept Introduction:
Atoms: Atoms consist of tiny particles called protons, neutrons and electrons. Proton and neutrons are present in the nucleus and the electron resides around the nucleus. The protons number will be same as the electrons count in the atom.
The symbol of an element is
Where,
(b)
Explanation of Solution
Given:
Radius of the nucleus is
Mass of the nucleus is
Calculation of density of the nucleus.
Consider, the nucleus is spherical, and the volume of the nucleus is:
From which density of the nucleus is,
In order to calculate the density, the value of volume is must; which is calculated as shown above by considering the nucleus is spherical.
Calculation of density of space occupied by electrons in sodium atom.
To be required: The mass of
Mass of 11 electrons are:
The volume occupied by the electron is obtained by the differences between the volume of the atom and volume of nucleus.
The volume of atom is:
Conversion of pm to cm:
The volume occupied by the nucleus is significant compared to the space occupied by the atoms.
Hence, the required terms are sufficient to calculate the density of the electrons:
The density of the electrons is
Want to see more full solutions like this?
Chapter 2 Solutions
Chemistry
- The photo here depicts what happens when a coil of magnesium ribbon and a few calcium chips are placed in water. (a) Based on these observations, what might you expect to see when barium, another Croup 2A element, is placed in water? (b) Give the period in which each element (Mg. Ca, and Ba) is found. What correlation do you think you might find between the reactivity of these elements and their positions in the periodic table?arrow_forwardThere are 2.619 1022 atoms in 1.000 g of sodium. Assume that sodium atoms are spheres of radius 1.86 and that they are lined up side by side. How many miles in length is the line of sodium atoms?arrow_forwardWhat evidence led to the conclusion that cathode rays had a negative charge?arrow_forward
- From the following written description, write the balanced chemical equation for the reaction including state symbols. A diatomic gaseous molecule that contains 17 protons per atom is reacted with a solid element that has an atomic number of 19 to yield an ionic compound.arrow_forwardA sample of metallic element X, weighing 3.177 g, combines with 0.6015 L of O2 gas (at normal pressure and 20.0C) to form the metal oxide with the formula XO. If the density of O2 gas under these conditions is 1.330 g/L, what is the mass of this oxygen? The atomic weight of oxygen is 15.999 amu. What is the atomic weight of X? What is the identity of X?arrow_forwardUsing the information in Table 2.1, answer the following questions. In an ion with an unknown charge, the total mass of all the electrons was determined to be 2.55 1026 g. while the total mass of its protons was 5.34 1023 g. What is the identity and charge of this ion? What is the symbol and mass number of a neutral atom whose total mass of its electrons is 3.92 1026 g, while its neutrons have a mass of 9.35 1023 g?arrow_forward
- In a hypothetical universe, an oil-drop experiment gave the following measurements of charges on oil drops: 5.55 1019 C, 9.25 1019 C, 1.11 1018 C, and 1.48 1018 C. Assume that the smallest difference in charge equals the unit of negative charge in this universe. What is the value of this unit of charge? How many units of excess negative charge are there on each oil drop?arrow_forward(a) Assuming the dimensions of the nucleus and atomshown in Figure 2.10, what fraction of the volume of the atomis taken up by the nucleus? (b) Using the mass of the protonfrom Table 2.1 and assuming its diameter is 1.0 x 10-15 m,calculate the density of a proton in g/cm3.arrow_forwardthe number of copper atoms in 3.0 g lump of copper is 2.8 x 1022. how many electrons are present in the sample? How much do they contribute to its mass?arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning





