
Biology 2e
2nd Edition
ISBN: 9781947172517
Author: Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 3VCQ
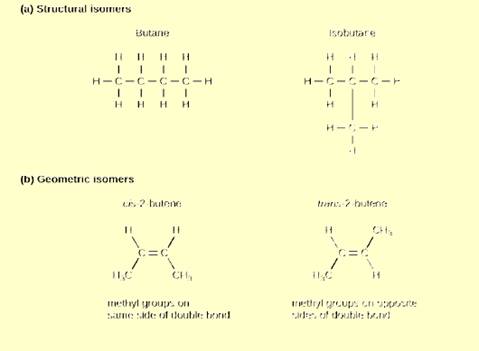
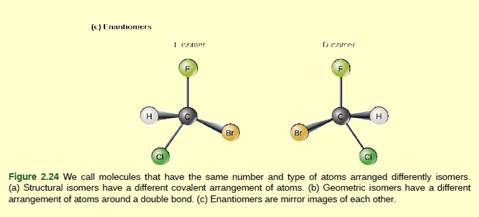
Figure 2.24 Which of the following statements is
false?
- Molecules with the formulas CH3CH2COOH and C3H6O2 could be structural isomers.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
The naturally-occurring amino acid L-serine (NH2-CH(COOH)-CH2-OH, dry form) shares all of the
following features with the naturally-occurring amino acid D-serine, except:
the same three-dimensional arrangement of covalent bonds
the same stoichiometry
the same number of covalent bonds
the exact same atoms
the same number of atoms
Which of the following is an example of a hydrogen bond?
the bond between the H of one water molecule and the O of another water molecule
the bond between C and H in methane
the bond between Na and Cl in salt
the bond between Mg and Cl in MgCl2
the bond between two hydrogen atoms
Which of the following statements is NOT true of biological organic molecules?
None of the other four answers (all are true)
They’re usually polymers formed from subunits (monomers)
Their atoms are mostly or entirely covalently bonded
Monomers are linked (bonded) together by hydrolysis (“water splitting”)
Always contain hydrogen and carbon
Chapter 2 Solutions
Biology 2e
Ch. 2 - Figure 2.3 How many neutrons do carbon-12 and...Ch. 2 - Figure 2.7 An atom may give, take, or share...Ch. 2 - Figure 2.24 Which of the following statements is...Ch. 2 - If xenon has an atomic number of 54 and a mass...Ch. 2 - Atoms that vary in the number of neutrons found in...Ch. 2 - Potassium has an atomic number of 19. What is its...Ch. 2 - Which type of bond represents a weak chemical...Ch. 2 - Which of the following statements is not true?...Ch. 2 - When acids are added to a solution, the pH should...Ch. 2 - We call a molecule that binds up excess hydrogen...
Ch. 2 - Which of the following statements is true? Acids...Ch. 2 - Each carbon molecule can bond with as many as...Ch. 2 - Which of the following is not a functional group...Ch. 2 - What makes ionic bonds different from covalent...Ch. 2 - Why are hydrogen bonds and van der Waals...Ch. 2 - Discuss how buffers help prevent drastic swings in...Ch. 2 - Why can some insects walk on water?Ch. 2 - What property of carbon makes it essential for...Ch. 2 - Compare and contrast saturated and unsaturated...
Additional Science Textbook Solutions
Find more solutions based on key concepts
The existence of the dwarf planet Pluto was proposed based on irregularities in Neptune's orbit. Pluto was subs...
College Physics
Pigeons may exhibit a checkered or plain color pattern. In a series of controlled matings, the following data w...
Concepts of Genetics (12th Edition)
Which type of cartilage is most plentiful in the adult body?
Anatomy & Physiology
In a mark-recapture study, an ecologist traps, marks, and releases 25 voles in a small wooded area. A week late...
Study Guide for Campbell Biology
QUANTITATIVE Punnett Squares as Genetic Tools. The genetic characters of seed color (where Y is the allele for ...
Becker's World of the Cell (9th Edition)
Compare and contrast aerobic respiration, anaerobic respiration, and fermentation.
Microbiology with Diseases by Body System (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- What kinds of bonds often control the shape (or tertiary form) of large molecules such as proteins? a. hydrogen b. ionic c. covalent d. inert e. singlearrow_forwardWhich of the following is a class of molecules that encompasses all of the other molecules listed? a. triglycerides b. fatty acids c. waxes d. steroids e. lipids f. phospholipidsarrow_forwardA carbon atom can form up ________ to bonds with other atoms. a. four b. six c. eight d. sixteenarrow_forward
- The backbone of organic compounds forms when _________ atoms are covalently bonded.arrow_forwardEach carbon atom can bond with as many as _________ other atoms.arrow_forwardFigure 2.12 A pH scale. Here, red dots signify hydrogen ions (H+) and blue dots signify hydroxyl ions (OH). Also shown are the approximate pH values for some common solutions. This pH scale ranges from 0 (most acidic) to 14 (most basic). A change of one unit on the scale corresponds to a tenfold change in the amount of H+ ions. Photos, JupiterImages Corporation. Figure It Out: What is the approximate pH of cola?arrow_forward
- Which type of bond represents a weak chemical bond? a. hydrogen bond b. ionic bond c. covalent bond d. polar covalent bondarrow_forwardWhich of the following types of bonds are present in the primary structures of proteins? Covalent bonds, hydrogen bonds and dislufide linkages Ionic interactions Hydrogen bonds and covalent bonds Covalent bondarrow_forwardIn a single molecule of water, the two hydrogen atoms are bonded to a single oxygen atom by nonpolar covalent bonds. polar covalent bonds. ionic bonds. hydrogen bonds. van der Waals interactionsarrow_forward
- Which covalent bond forms when a fatty acid is attached to the following molecule by dehydration synthesis: CH2(OH)-CH(OH)-CH2(OH)? a peptide bond a phosphodiester bond an ester bond a glycosidic bond an ether bondarrow_forwardMonomers are linked together by removing a __ from one monomer, and a __ from another monomer. hydroxyl group; hydrogen hydroxyl group; hydroxyl group carboxyl group; hydrogen carboxyl group; phosphate hydroxyl group; carboxyl grouparrow_forwardThe type of molecule in the above question is the MONOMER of which of the following polymers? Lipids Polysaccharides Nucleic acids Proteinsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Biology (MindTap Course List)
Biology
ISBN:9781337392938
Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:Cengage Learning

Biology Today and Tomorrow without Physiology (Mi...
Biology
ISBN:9781305117396
Author:Cecie Starr, Christine Evers, Lisa Starr
Publisher:Cengage Learning

Human Biology (MindTap Course List)
Biology
ISBN:9781305112100
Author:Cecie Starr, Beverly McMillan
Publisher:Cengage Learning

Concepts of Biology
Biology
ISBN:9781938168116
Author:Samantha Fowler, Rebecca Roush, James Wise
Publisher:OpenStax College

Macromolecules | Classes and Functions; Author: 2 Minute Classroom;https://www.youtube.com/watch?v=V5hhrDFo8Vk;License: Standard youtube license