
Concept explainers
Interpretation:
The structural formula for 2-propanol should be drawn and whether it is primary, secondary, or tertiary alcohol should be indicated.
Concept Introduction:
The formula in which the arrangements of atoms in a molecule are shown is said to be the structural formula.
- An organic compound in which hydroxyl functional group that is -OH is bonded to the carbon atom is said to be an alcohol. The general formula for alcohol is Cn H2 n + 1 OH. Based on the attachment to the carbon the alcohols are classified as primary, secondary, or tertiary alcohol.
When alcohol (having hydroxyl group) is attached to a primary carbon atom (carbon atom to which one carbon atom is attached) then such alcohol is said to be a primary alcohol.
When alcohol (having hydroxyl group) is attached to a secondary carbon atom (carbon atom to which two carbon atoms are attached) then such alcohol is said to be a secondary alcohol.
When alcohol (having hydroxyl group) is attached to a tertiary carbon atom (carbon atom to which three carbon atoms are attached) then such alcohol is said to be a tertiary alcohol.
Answer to Problem 137AP
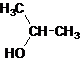
Secondary alcohol.
Explanation of Solution
For number of carbons atoms in chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
The numbering of the carbon chain is done in such a way that the substituents get the lower number.
The given name is 2-propanol that means the parent chain has 3 number of carbons in the chain and suffix -ol represents it is an alcohol group that is R-OH (where R is
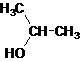
Since, -OH is attached to secondary carbon so, it is a secondary alcohol.
Interpretation:
The structural formula for 2-methyl-2-propanol should be drawn and whether it is primary, secondary, or tertiary alcohol should be indicated.
Concept Introduction:
The formula in which the arrangements of atoms in a molecule are shown is said to be the structural formula.
- An organic compound in which hydroxyl functional group that is -OH is bonded to the carbon atom is said to be an alcohol. The general formula for alcohol is Cn H2 n + 1 OH. Based on the attachment to the carbon the alcohols are classified as primary, secondary, or tertiary alcohol.
When alcohol (having hydroxyl group) is attached to a primary carbon atom (carbon atom to which one carbon atom is attached) then such alcohol is said to be a primary alcohol.
When alcohol (having hydroxyl group) is attached to a secondary carbon atom (carbon atom to which two carbon atoms are attached) then such alcohol is said to be a secondary alcohol.
When alcohol (having hydroxyl group) is attached to a tertiary carbon atom (carbon atom to which three carbon atoms are attached) then such alcohol is said to be a tertiary alcohol.
Answer to Problem 137AP
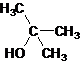
Tertiary alcohol.
Explanation of Solution
For number of carbons atoms in chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
The numbering of the carbon chain is done in such a way that the substituents get the lower number.
The given name is 2-methyl-2-propanol that means the parent chain has 3 number of carbons in the chain and suffix -ol represents it is an alcohol group that is R-OH (where R is aryl/alkyl group) at 2 position, and a methyl substitution at 2 position. So, the structural formula of 2-methyl-2-propanol is:
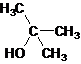
Since, -OH is attached to tertiary carbon so, it is a tertiary alcohol.
Interpretation:
The structural formula for 4-isopropyl-2-heptanol should be drawn and whether the it is primary, secondary, or tertiary alcohol should be indicated.
Concept Introduction:
The formula in which the arrangements of atoms in a molecule are shown is said to be the structural formula.
- An organic compound in which hydroxyl functional group that is -OH is bonded to the carbon atom is said to be an alcohol. The general formula for alcohol is Cn H2 n + 1 OH. Based on the attachment to the carbon the alcohols are classified as primary, secondary, or tertiary alcohol.
When alcohol (having hydroxyl group) is attached to a primary carbon atom (carbon atom to which one carbon atom is attached) then such alcohol is said to be a primary alcohol.
When alcohol (having hydroxyl group) is attached to a secondary carbon atom (carbon atom to which two carbon atoms are attached) then such alcohol is said to be a secondary alcohol.
When alcohol (having hydroxyl group) is attached to a tertiary carbon atom (carbon atom to which three carbon atoms are attached) then such alcohol is said to be a tertiary alcohol.
Answer to Problem 137AP
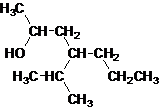
Secondary alcohol.
Explanation of Solution
For number of carbons atoms in chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
The numbering of the carbon chain is done in such a way that the substituents gets the lower number.
The given name is 4-isopropyl-2-heptanol that means the parent chain has 7 number of carbons in the chain and suffix -ol represents it is an alcohol group that is R-OH (where R is aryl/alkyl group) at 2 position, and anisopropyl substitution at 4 position. So, the structural formula of 4-isopropyl-2-heptanol is:
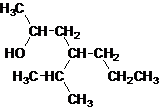
Since, -OH is attached to secondary carbon so, it is a secondary alcohol.
Interpretation:
The structural formula for 2, 3-dichloro-1-pentanol should be drawn and whether the it is primary, secondary, or tertiary alcohol should be indicated.
Concept Introduction:
The formula in which the arrangements of atoms in a molecule are shown is said to be the structural formula.
- An organic compound in which hydroxyl functional group that is -OH is bonded to the carbon atom is said to be an alcohol. The general formula for alcohol is Cn H2 n + 1 OH. Based on the attachment to the carbon the alcohols are classified as primary, secondary, or tertiary alcohol.
When alcohol (having hydroxyl group) is attached to a primary carbon atom (carbon atom to which one carbon atom is attached) then such alcohol is said to be a primary alcohol.
When alcohol (having hydroxyl group) is attached to a secondary carbon atom (carbon atom to which two carbon atoms are attached) then such alcohol is said to be a secondary alcohol.
When alcohol (having hydroxyl group) is attached to a tertiary carbon atom (carbon atom to which three carbon atoms are attached) then such alcohol is said to be a tertiary alcohol.
Answer to Problem 137AP
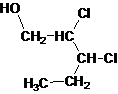
Primary alcohol.
Explanation of Solution
For number of carbons atoms in chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
The numbering of the carbon chain is done in such a way that the substituents get the lower number.
The given name is 2, 3-dichloro-1-pentanol that means the parent chain has 5 number of carbons in the chain and suffix -ol represents it is an alcohol group that is R-OH (where R is aryl/alkyl group) at 1 position, and 2 chlorine substitution at 2 and 3 position. So, the structural formula of 2, 3-dichloro-1-pentanol is:
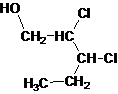
Since, -OH is attached to primary carbon so, it is a primary alcohol.
Want to see more full solutions like this?
Chapter 20 Solutions
Introductory Chemistry: A Foundation
- The general formula of an alkane is CnH2n+2 . What is the general formula of an (a) alkene? (b) alkyne? (c) alcohol derived from an alkane?arrow_forwardFor each of the following alcohols, give the systematic name and specify whether the alcohol is primary, secondary, or tertiary. a. b. c.arrow_forwardHow would you synthesize each of the following?a. 1,2-dibromopropane from propeneb. acetone (2-propanone) from an alcoholc. tert-butyl alcohol (2-methyl-2-propanol) from an alkene (See Exercise 62.)d. propanoic acid from an alcoholarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





