
Concept explainers
(a)
Interpretation:
The product formed when cholesterol reacts with hydrogen in presence of palladium-carbon has to be drawn.
(a)
Explanation of Solution
Cholesterol with hydrogen:
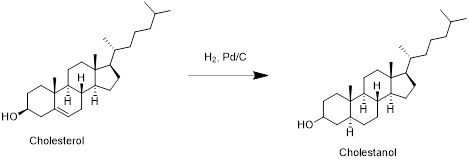
Cholesterol reacts with hydrogen in presence of palladium-carbon to reduce double bond to single bond and forms cholestanol. Therefore, the product obtained is cholestanol which is shown in the above scheme.
(b)
Interpretation:
The product formed when cholesterol reacts with acetyl chloride has to be drawn
(b)
Explanation of Solution
Cholesterol with acetyl chloride:
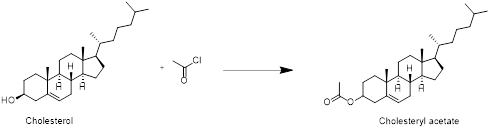
Cholesterol reacts with acetyl chloride in which acetylation reaction take place at hydroxyl group and forms cholesteryl acetate. Therefore, the product obtained is cholesteryl acetate which is shown in the above scheme.
(c)
Interpretation:
The product formed when cholesterol reacts with sulfuric acid followed by heating has to be drawn.
(c)
Explanation of Solution
Cholesterol with sulfuric acid:
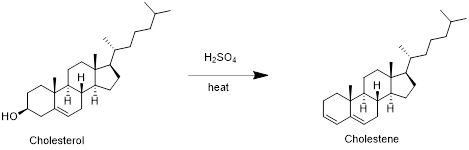
Cholesterol reacts with sulfuric acid in which removal of water molecule take place and forms cholestene. Therefore, the product obtained is cholestene which is shown in the above scheme.
(d)
Interpretation:
The product formed when cholesterol reacts with water in presence of acid has to be drawn.
(d)
Explanation of Solution
Cholesterol with water:
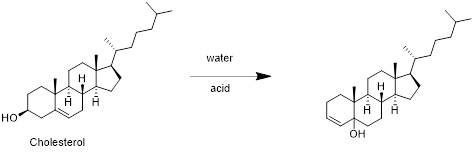
Cholesterol reacts with water in presence of water in which removal of water molecule take place and forms desired product. Therefore, the product obtained is is shown in the above scheme.
(e)
Interpretation:
The product formed when cholesterol reacts with peroxy acid has to be drawn
(e)
Explanation of Solution
Cholesterol with peroxy acid:
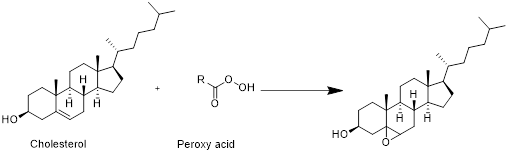
Cholesterol reacts with peroxy acid in presence of water in which reaction take place at double bond and forms
Want to see more full solutions like this?
Chapter 20 Solutions
Essential Organic Chemistry, Global Edition
- What is the role of concentrated H2SO4 in the esterification reaction?arrow_forwardHow many products are obtained when the phospholipid below is saponified? a. 4 b. 6 c. 2 d. 3 e. 5arrow_forwardWhat is the final product and product name when you mix 4-nitrobenzaldehyde with each of these: a) aminoguanidine bicarbonate b) 4-chlorophenylhydrazine hydrochloridearrow_forward
- Caused by the severe dehydration effect of formaldehyde on tissue A. fumigation B. hydration C. searing D. soaponificationarrow_forwardShow the saponification of a solid soap.arrow_forwardCeramide can then be used to synthesize sphingomyelin, another important molecule that is prevalent in cell membranes. 1.How does the structure of sphingomyelin differ from that of ceramide? 2.Sphingomyelin is also found in the cell membrane. Explain how you would expect to find the orientation of this molecule in the cell membrane. 3.Which amino alcohol is used in the synthesis of sphingomyelin? 4.What is particular about the amino alcohol used in in the synthesis of sphingomyelin? 5.How does your answer to #16 affect the physical properties of properties of sphingomyelin?arrow_forward
- draw the product of cyclohexanone and morpholine.arrow_forwardGive the functions of the following ingredients, then name a branded/commercial skin or hair care product where the said material is used. Tetrasodium EDTA Cholesterol Silicone Oil 200/50 cst BETAINE Carbopol Ultrez D & C Red No. 17 ( CI No. 26100) Bisabolol (Alpha Bisabolol) Ceresin Wax Disodium Lauryl Sulfosuccinate Hydroxypropyl Methylcellulosearrow_forwardDraw the products of the diethyl heptanedioate: (1) sodium ethoxide; (2) HClarrow_forward
- Synthesising menthone is an exothermic process that includes a reflux procedure. Mark the correct statements. * A- The reflux process enables us to transfers energy to the reaction mixture for an extended period of time without loss of solvent or reagents. B- The reflux process increases the rate of the oxidation of menthol. C- The reflux process increases the yield of the oxidation of menthol. D- A water bath can be used in the preparation of menthone because the boiling point of the reaction mixture is below 100°C due to the inclusion of acetone in this mixture.arrow_forward1. What test reagents are used for the alkylation of amino acids (alkylating reagents)? 2. What test reagents are used for acid hydrolyzation of amino acidsarrow_forwardShow the saponification of a liquid soap.arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
