
Concept explainers
What reagent is needed to convert
a. 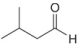 b.
b. 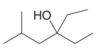 c.
c. 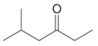 d.
d. 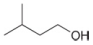
(a)
Interpretation: The reagent needed to convert
Concept introduction: Acid chlorides, which contains good leaving group
Answer to Problem 20.30P
The reagent needed to convert
Explanation of Solution
The given compound is aldehyde. Acid chlorides on reaction with mild reducing agent like lithium tri-tert-butoxyaluminium hydride
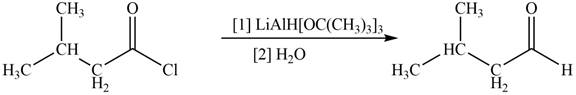
Figure 1
Therefore,
The reagent needed to convert
(b)
Interpretation: The reagent needed to convert
Concept introduction: Acid chlorides, which contains good leaving group
Answer to Problem 20.30P
The reagent needed to convert
Explanation of Solution
The structure of given ketone compound shows that the hydroxyl group is attached to tertiary carbon atom. Acid chlorides on reaction with two equivalents of Grignard reagent convert into tertiary alcohol. The first equivalent of Grignard reagent remove good leaving
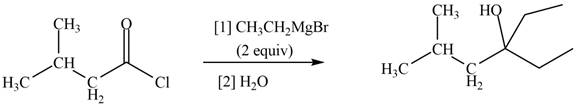
Figure 2
Therefore,
The reagent needed to convert
(c)
Interpretation: The reagent needed to convert
Concept introduction: Acid chlorides, which contains good leaving group
Answer to Problem 20.30P
The reagent needed to convert
Explanation of Solution
The structure of given compound shows that the carbonyl carbon is attached to ethyl group. Ethyl group can occupy the position of leaving group
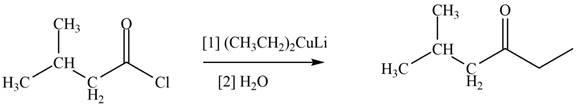
Figure 3
Therefore,
The reagent needed to convert
(d)
Interpretation: The reagent needed to convert
Concept introduction: Acid chlorides, which contains good leaving group
Answer to Problem 20.30P
The reagent needed to convert
Explanation of Solution
Acid chlorides on reaction with strong reducing agent like lithium aluminium hydride
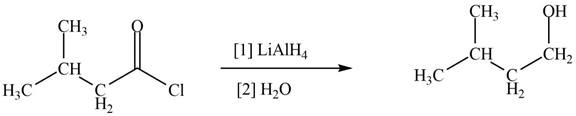
Figure 4
Therefore,
The reagent needed to convert
Want to see more full solutions like this?
Chapter 20 Solutions
Organic Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





