
(a)
Interpretation: For the chair-like transition state of a
Concept Introduction:
It is a special class of pericyclic reaction in which a
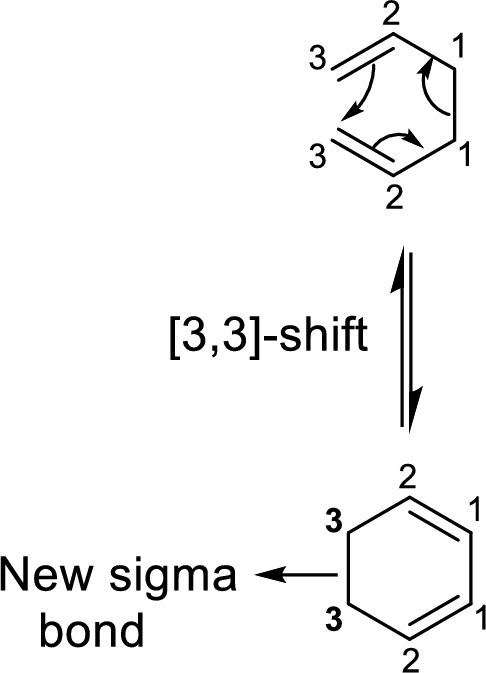
Stereochemistry in
Two types of transition states are likely to occur in this shift such as chair-like and boat-like transition states. According to the Frontier-Molecular-Orbital theory, both the transition states are allowed transition states inspite of the stability difference. The product obtained from the chair-like transition state has trans-conformation whereas the product obtained from the boat-like transition state has cis-conformation.
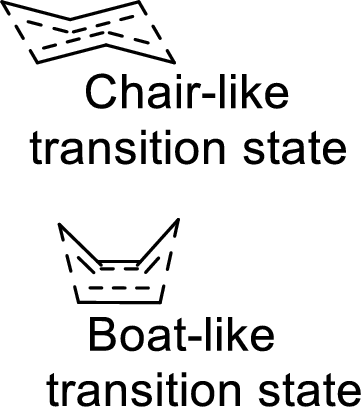
(b)
Interpretation: The reason for why the products with boat-like conformation are formed to a lower extent than those with chair-like conformation.
Concept Introduction:
It is a special class of pericyclic reaction in which a
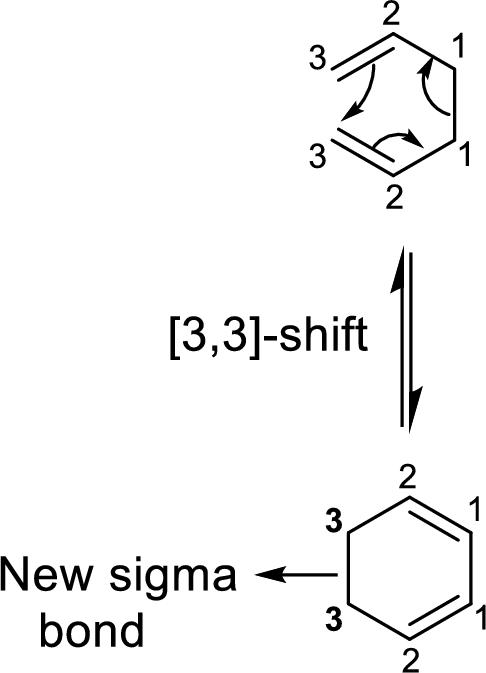
Stereochemistry in
Two types of transition states are likely to occur in this shift such as chair-like and boat-like transition states. According to the Frontier-Molecular-Orbital theory, both the transition states are allowed transition states inspite of the stability difference. The product obtained from the chair-like transition state has trans-conformation whereas the product obtained from the boat-like transition state has cis-conformation.
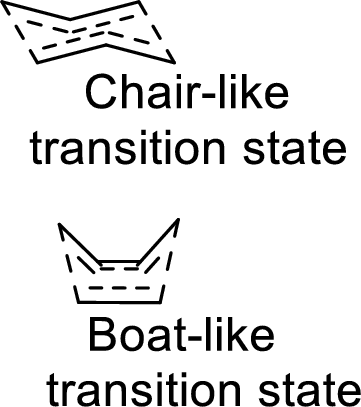
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
Organic Chemistry
- Consider the reaction of two compounds ‘A’ and ‘B’ which could make two possible diastereomers ‘AB’ and ‘BA’ (much like this week’s Diels Alder reaction). Hand-write your calculations and responses to the following questions and upload your work as a .jpg or .pdf file. Which of the two products (A or B) will form in greater abundance under thermodynamic control? Which will form in greater abundance under kinetic control? Explain your responses using a sketch of the reaction coordinate diagram for the reactions described above.arrow_forward. Discuss the truth of the following statement. Explain why it is true or false Every SN1 reaction produces racemic mixtures in the productsarrow_forwardDraw all the resonance forms of the sigma complex for nitration of bromobenzene at the ortho, meta, and para positions. Point out why the intermediate for meta substitution is less stable than the other two.arrow_forward
- A chemist is attempting to synthesize a complex natural product with a highly strained cyclohexene ring system. Which type of reactants would be most suitable for achieving this goal, and why? Provide a detailed explanation of the choice of reactants and the expected outcome in terms of the Diels-Alder reaction.arrow_forwardExplain and give examples of the stability order of carbocations within the group and among different groupsarrow_forwardThe molecular formula of unknown compound B is C10H16.Quantitative brominationof a 0.473-g sample of compound Brequired 48.22mL of a 0.216MBr2/CCl4to produce a color change. How many rings and pi bonds does compound Bcontain?Include any explanations/calculations to justify your answers.arrow_forward
- Discuss comprehensively whether the circledfunctional group is an activator or deactivatorduring SEArarrow_forwardDiisopinocampheylborane (Ipc2BH) is a chiral organoborane, readily employed for the production of many asymmetric products used in total synthesis. It is a crystalline material that can be prepared as a single enantiomer via the hydroboration of two equivalents of α-pinene with borane.Explain why only one enantiomer of Ipc2BH is formed.arrow_forwardDiscuss comprehensively why -Cl and -NO2 aredeactivators and why -Cl is a weak and -NO2 astrong deactivator?arrow_forward
- give some real life application of stereochemistryarrow_forwardWhat are some of the experimental things that can be done to help control the Friedel-Crafts alkylationsreactionarrow_forwardA conjugated diene with an even number of double bonds undergoes conrotatory ring closure underthermal conditions.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


