
Concept explainers
Draw the products formed when
(a)
Interpretation: The product formed by the treatment of
Concept introduction: Treatment of carbonyl compounds with
Answer to Problem 20.7P
The product formed by the treatment of
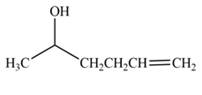
Explanation of Solution
The reduction of ketone by
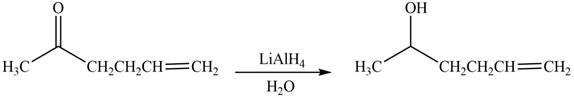
Figure 1
The product formed by the treatment of
(b)
Interpretation: The product formed by the treatment of
Concept introduction: Treatment of carbonyl compounds with
Answer to Problem 20.7P
The product formed by the treatment of
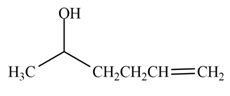
Explanation of Solution
The reduction of ketone by
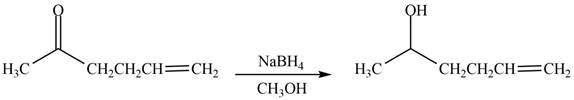
Figure 2
The product formed by the treatment of
(c)
Interpretation: The product formed by the treatment of
Concept introduction: Treatment of carbonyl compounds with
Answer to Problem 20.7P
The product formed by the treatment of
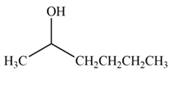
Explanation of Solution
Treatment of carbonyl compounds with
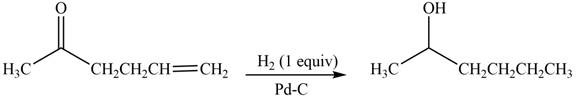
Figure 3
The product formed by the treatment of
(d)
Interpretation: The product formed by the treatment of
Concept introduction: Treatment of carbonyl compounds with
Answer to Problem 20.7P
The product formed by the treatment of
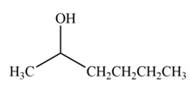
Explanation of Solution
Treatment of carbonyl compounds with
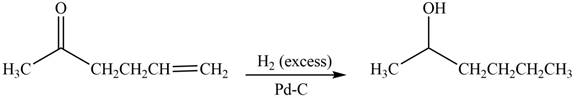
Figure 4
The product formed by the treatment of
(e)
Interpretation: The product formed by the treatment of
Concept introduction: Treatment of carbonyl compounds with
Answer to Problem 20.7P
The product formed by the treatment of
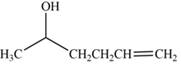
Explanation of Solution
The reduction of ketone by
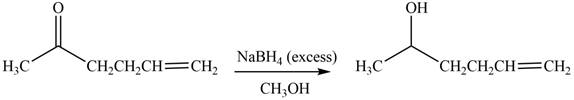
Figure 5
The product formed by the treatment of
(f)
Interpretation: The product formed by the treatment of
Concept introduction: Treatment of carbonyl compounds with
Answer to Problem 20.7P
The product formed by the treatment of
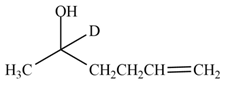
Explanation of Solution
The reduction of ketone by
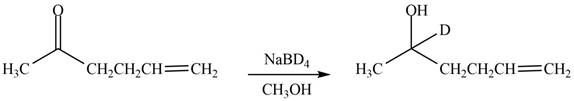
Figure 6
The product formed by the treatment of
Want to see more full solutions like this?
Chapter 20 Solutions
Organic Chemistry
- Eleostearic acid, C18H30O2, is a rare fatty acid found in the tung oil used for finishing furniture. On ozonolysis followed by treatment with zinc, eleostearic acid furnishes one part pentanal, two parts glyoxal (OHC-CHO), and one part 9-oxononanoic acid [OHC(CH2)7CO2H]. What is the structure of eleostearic acid?arrow_forwardDraw a resonance structure of the acetonitrile anion, -: CH2CN, and account for the acidity of nitriles.arrow_forwardAxial alcohols are oxidized faster than equatorial alcohols by PCC andother Cr6+ oxidants. Which OH group in each compound is oxidizedfaster?arrow_forward
- A reaction of an alkene plus H2O and excess acid.arrow_forwardDraw the product formed when (CH3)2CHOH is treated with each reagent. a. SOCl2, pyridine b. TsCl, pyridine c. H2SO4 d. HBr e. PBr3, then NaCN f. POCl3, pyridinearrow_forwardThe key step in a reported laboratory synthesis of sativene, a hydrocarbon isolated from the mold Helminthosporium sativum, involves the following base treatment of a keto tosylate. What kind of reaction is occurring? How would you complete the synthesis?arrow_forward
- Draw the organic product(s) formed upon the addition of HBr to (a) 2-methyl-2-pentene, (b) trans-2-hexene, and (c) 4-methylcyclohexene. How many regioisomers can be formed in each case?arrow_forwardDraw the products 1. (S)-2-chlorobutane and sodium acetate in DMSO 2. 1-bromopropane and methylamine in acetonitrile.arrow_forwardDraw all of the substitution and elimination products formed from the given alkyl halide with each reagent: (a) CH3OH; (b) KOH. Indicate the stereochemistry around the stereogenic centers present in the products, as well as the mechanism by which each product is formed.arrow_forward
- Draw the products formed when (CH3)2C=CH2 is treated with following reagent. [1] BH3; [2] H2O2, HO−arrow_forward(a) 2,4-dicyclopropyl-1-phenylpentane (b) 1,4-dichlorobenzene (c) (3E) hept-3-enoic acid (d) ethyl 2-methylbutanoate (e) 2-methoxypropan-1-ol (f) oct-4-ynal (g) 1,1-dichloro-2,2-difluoroethane (h) trimethylamine (i) (4Z) hex-4-en-2-ol (j) N-methylpropanamide draw structuresarrow_forwardDraw the products formed when (CH3)2C=CH2 is treated with each reagent.a. HBrb. H2OH2SO4c. CH3CH2OH, H2SO4d. Cl2e. Br2, H2Of. NBS (aqueous DMSO)g. [1]BH3;[2]H2O2, HO-arrow_forward
