
Chemistry
9th Edition
ISBN: 9781133611097
Author: Steven S. Zumdahl
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 20, Problem 35E
The following illustration shows the orbitals used to form the bonds in carbon dioxide.
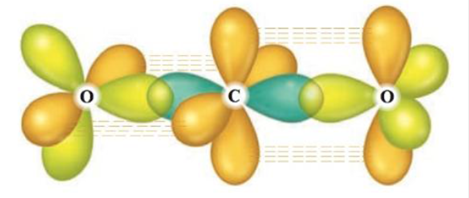
Each color represents a different orbital. Label each orbital, draw the Lewis structure for carbon dioxide, and explain how the localized electron model describes the bonding in CO2.
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 20 Solutions
Chemistry
Ch. 20 - What are the two most abundant elements by mass in...Ch. 20 - Prob. 2RQCh. 20 - Prob. 3RQCh. 20 - What is the valence electron configuration for the...Ch. 20 - Prob. 5RQCh. 20 - Prob. 6RQCh. 20 - Table 19-14 lists some common nitrogen compounds...Ch. 20 - Prob. 8RQCh. 20 - Prob. 9RQCh. 20 - Prob. 10RQ
Ch. 20 - Prob. 1QCh. 20 - Prob. 2QCh. 20 - Prob. 3QCh. 20 - Diagonal relationships in the periodic table exist...Ch. 20 - Prob. 6QCh. 20 - Prob. 7QCh. 20 - Prob. 8QCh. 20 - All the Group 1A (1) and 2A (2) metals are...Ch. 20 - Prob. 10QCh. 20 - Prob. 13ECh. 20 - Prob. 14ECh. 20 - Prob. 15ECh. 20 - Prob. 16ECh. 20 - Prob. 17ECh. 20 - Prob. 18ECh. 20 - Prob. 19ECh. 20 - Prob. 20ECh. 20 - Prob. 21ECh. 20 - Electrolysis of an alkaline earth metal chloride...Ch. 20 - Prob. 24ECh. 20 - Prob. 25ECh. 20 - Prob. 26ECh. 20 - Boron hydrides were once evaluated for possible...Ch. 20 - Prob. 28ECh. 20 - Prob. 29ECh. 20 - Prob. 30ECh. 20 - Prob. 31ECh. 20 - Prob. 32ECh. 20 - Prob. 33ECh. 20 - Prob. 34ECh. 20 - The following illustration shows the orbitals used...Ch. 20 - Prob. 36ECh. 20 - Silicon is produced for the chemical and...Ch. 20 - Prob. 38ECh. 20 - The compound Pb3O4 (red lead) contains a mixture...Ch. 20 - Prob. 40ECh. 20 - Prob. 41ECh. 20 - Prob. 42ECh. 20 - Prob. 43ECh. 20 - Prob. 44ECh. 20 - Prob. 45ECh. 20 - Prob. 46ECh. 20 - Prob. 47ECh. 20 - Prob. 48ECh. 20 - Prob. 49ECh. 20 - Phosphate buffers are important in regulating the...Ch. 20 - Prob. 51ECh. 20 - Trisodium phosphate (TSP) is an effective grease...Ch. 20 - Prob. 53ECh. 20 - Prob. 54ECh. 20 - Prob. 55ECh. 20 - Complete and balance each of the following...Ch. 20 - Prob. 57ECh. 20 - Prob. 58ECh. 20 - How can the paramagnetism of O2 be explained using...Ch. 20 - Describe the bonding in SO2 and SO3 using the...Ch. 20 - Write the Lewis structure for O2F2. Predict the...Ch. 20 - Give the Lewis structure, molecular structure, and...Ch. 20 - Prob. 63ECh. 20 - Prob. 64ECh. 20 - Prob. 65ECh. 20 - Prob. 66ECh. 20 - Prob. 67ECh. 20 - Prob. 68ECh. 20 - Prob. 69ECh. 20 - Prob. 70ECh. 20 - Prob. 71ECh. 20 - Prob. 72ECh. 20 - Prob. 73AECh. 20 - The inert-pair effect is sometimes used to explain...Ch. 20 - How could you determine experimentally whether the...Ch. 20 - Prob. 76AECh. 20 - Prob. 77AECh. 20 - Prob. 78AECh. 20 - Prob. 79AECh. 20 - Draw Lewis structures for the AsCl4+ and AsCl6...Ch. 20 - Prob. 81AECh. 20 - Prob. 82AECh. 20 - Prob. 83AECh. 20 - What is a disproportionation reaction? Use the...Ch. 20 - Sulfur forms a wide variety of compounds in which...Ch. 20 - Prob. 86AECh. 20 - Prob. 87CWPCh. 20 - Prob. 88CWPCh. 20 - Prob. 89CWPCh. 20 - Prob. 90CWPCh. 20 - Prob. 91CWPCh. 20 - Nitrous oxide (N2O) can be produced by thermal...Ch. 20 - What is the hybridization of the central atom in...Ch. 20 - Prob. 94CWPCh. 20 - Prob. 95CWPCh. 20 - Prob. 96CWPCh. 20 - Prob. 97CPCh. 20 - Prob. 98CPCh. 20 - Lead forms compounds in the +2 and +4 oxidation...Ch. 20 - Prob. 100CPCh. 20 - Prob. 101CPCh. 20 - Prob. 102CPCh. 20 - You travel to a distant, cold planet where the...Ch. 20 - Prob. 104CPCh. 20 - Prob. 105CPCh. 20 - Prob. 106IPCh. 20 - Prob. 107IPCh. 20 - Although nitrogen trifluoride (NF3) is a thermally...Ch. 20 - While selenic acid has the formula H2SeO4 and thus...Ch. 20 - Prob. 110MPCh. 20 - Prob. 111MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write Lewis structures for NF3 and PP5. On the basis of hybrid orbitals, explain the fact that NF3, PP3, and PP5 are stable molecules, but NP5 does not exist.arrow_forwardXenon trioxide, XeO3, is reduced to xenon in acidic solution by iodide ion. Iodide ion is oxidized to iodine, I2. Write a balanced chemical equation for the reaction.arrow_forward
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
NEET Chemistry | Group 14 Carbon Family | Theory & Problem Solving | In English | Misostudy; Author: Misostudy;https://www.youtube.com/watch?v=enOGIrcHh54;License: Standard YouTube License, CC-BY