
Concept explainers
(a)
Interpretation:
Complete the given
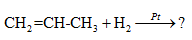
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms.
The chemical reaction which involves the addition of two hydrogen atoms across the double bond in the presence of catalyst is known as hydrogenation.
(b)
Interpretation:
Complete the given chemical reaction of alkene.
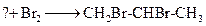
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms. Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
The chemical reaction which involves the addition of two halogens across the double bond is known as halogenation.
(c)
Interpretation:
Complete the given chemical reaction of alkene.
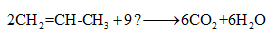
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms. Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
The reaction in which burning of hydrocarbons takes place in oxygen to release water and carbon dioxide is known combustion reaction of hydrocarbon.
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
Introductory Chemistry: A Foundation
- Name each of the following alkenes. a. CH2 = CH CH2 CH3 b. c.arrow_forwardThe reaction of an alkene with water produces what type of product? A) alkane B) alkyne C) alcohol D) aldehyde E) amino acidarrow_forwardComplete the following organic reactions and draw structural diagrams to represent the products. a) CH3CH(CH3)CH2COOCH3 + H2O → b) methyl propene + HCl(aq) →arrow_forward
- In a hydrogenation reaction of an alkene, hydrogen atoms are added to a double bond to form an __________. A) alkane B) alkene C) alkyne D) aromatic ringarrow_forwardConsider the structure in the attached picture: 1. the carbon where OH is attached is classified as a: a. primary b. secondary c. tertiary 2. When the compound is reacted with a dehydrating reagent like H2SO4, the compound formed will be a/an . a. alkane b. alkene c. aldehyde d. ketone 3. The product formed from its dehydration using sulfuric acid is _______. a. butane b. 2-butene c. butanal d. 2-butanonearrow_forward1. Organic compounds composed of carbon and hydrogen atoms connected by triple bonds are called_____ A. alkanes B. alkynes C. None of the others D. alkenes E. aromatic compoundarrow_forward
- Which of the following statements about Figure 26 is not true? * A- The two products are formed by two competing chemical reactions. B- The alcohol is formed in the greatest amount. C- The alcohol is formed by an Sɴ1mechanism. D- The alkene is formed by an E1 mechanism. E- All of these statements are correct.arrow_forwardWhat are fats and oils? a. ketones formed of two or more esters b. any large molecular weight organic compound that has been saturated c. esters formed from glycerol and three carboxylic acids d. polymers of repeating aromatic hydrocarbonsarrow_forward
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





