
Concept explainers
Suggest a reasonable explanation for each of the following observations:
The second-order rate constant
The second-order rate constant for saponification of ethyl
The second-order rate constant
The second-order rate constant
The second-order rate constant
The second-order rate constant
Interpretation:
The reasonable explanation for given observations is to be stated.
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction.
In nucleophilic substitution reaction, nucleophile takes the position of leaving group by attacking on the electron deficient carbon atom.
Answer to Problem 45P
Solution:
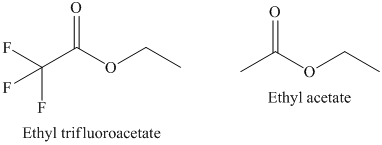
a) The rate constant for saponification of ethyl trifluoroacetate is more than ethyl acetate because the anionic intermediate is stabilized in ethyl trifluoroacetate.
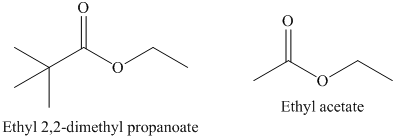
b) The rate constant for saponification of ethyl acetate is more than ethyl
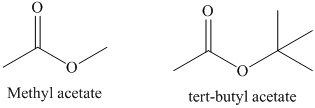
c) The rate constant for saponification of methyl acetate is more than tert-butyl acetate because the incoming nucleophile is less sterically hindered in methyl acetate.
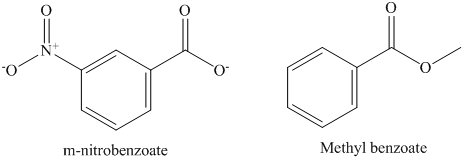
d) The rate constant for saponification of methyl m-nitrobenzoate is more than methyl benzoate because the anionic intermediate is stabilized in methyl m-nitrobenzoate.
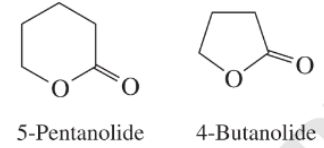
e) The rate constant for saponification of
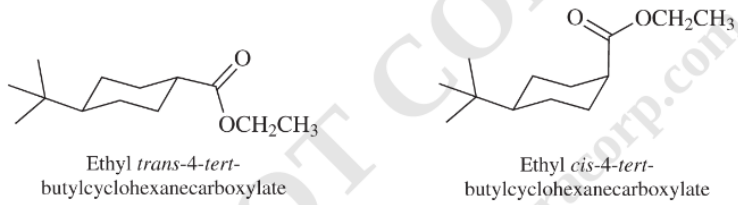
f) The rate constant for saponification of ethyl
Explanation of Solution
a) The saponification of esters involves cleavage of
In ethyl trifluoroacetate,
b) The saponification of esters involves cleavage of
In ethyl acetate,
c) The saponification of esters involves cleavage of
In the first step of saponification reaction, the incoming nucleophile attack on the carbonyl carbon and generate anionic intermediate. In tert-butyl acetate, the attack of incoming nucleophile is sterically hindered by the presence of three methyl groups. However, in methyl acetate only one methyl group is present. Therefore, the second order rate constant for saponification of methyl acetate is much higher than tert-butyl acetate. The structure of methyl acetate and tert-butyl acetate is shown below.
d) The saponification of esters involves cleavage of
In methyl m-nitrobenzoate, nitro group is attached to benzene ring which withdraws the electron density and stabilizes the intermediate. However, in methyl benzoate electron withdrawing group is not attached to benzene ring. It stabilizes anionic intermediate less than the methyl m-nitrobenzoate. Therefore, the second order rate constant for saponification of methyl m-nitrobenzoate is more than methyl benzoate. The structure of methyl m-nitrobenzoate and methyl benzoate is shown below.
e) The saponification of esters involves cleavage of
The anionic intermediate formed by the saponification of
f) The saponification of esters involves cleavage of
In ethyl
Want to see more full solutions like this?
Chapter 20 Solutions
Organic Chemistry - Standalone book
- Predict the products and mechanisms of the following reactions. When if more than one product or mechanism is possible, explain are the most probable. (d) 2-chloro-2-methylbutane by heating in ethanol (e) isobutyl iodide, KOH in ethanol / waterarrow_forwardFor the gas phase isomerization of isopropenyl allyl ether, CH2=C(CH3)-O-CH2CH=CH2-->CH3COCH2CH2CH=CH2 the rate constant at 405 K is 2.60x10^-5 s^-1 and the rate constant at 440. K is 4.75x10^-4 s^-1. The activation energy for the gas phase isomerization of isopropenyl allyl ether is ________ kJ.arrow_forwardsuggest a mechanism for this reaction. identify the type of molceule created by this reaction. identify the optimal solvent conditions that create the fasted reaction ratearrow_forward
- Predict the products and mechanisms of the following reactions. When more than oneproduct or mechanism is possible, explain which are most likely. ) 1@bromo@1@methylcyclopentane + NaOEt in ethanolarrow_forwardDescribe the reaction mechanism for the hydrolysis of 2-bromo-2-methylpropane [CH3C(CH3)2Br] with aqueous hydroxide ions (OH-).arrow_forwardOptically pure Compound 1 undergoes a reaction at room temperature with sodium methoxide (NaOCH3) in methanol to form a single isomer of Compound 2 as shown below: 1. What are the stereochemical designations (R or S) of Compound 1 and Compound 2? 2. On the basis of the structure of Compound 2 and the information on the reaction conditions, suggest which type of mechanism Compound 1 undergoes . 3. The rate of the above reaction is determined experimentally to follow second-order kinetics. Give a fully labelled sketch of a reaction coordinate diagram for the reaction. 4. draw a mechanism on a piece of paper (using curly arrows) to show the formation of Compound 2 from Compound 1 including any activated complex. 5. If the sodium methoxide is left out of the reaction mixture, Compound 2 is formed in roughly equal amounts with another compound (Compound 3). Suggest a structure for Compound 3. With reference to the mechanism of the reaction and the structure of Compound 1, explain how these…arrow_forward
- Under milder temperatures than what is needed for dehydration, strong acids catalyze the self-condensation of 1-propyl alcohol to give di-n-propyl ether. The mechanism differs from that of today's reaction in that the protonated alcohol undergoes an Sn2 rather than an Sn1 reaction. Write the mechanism for the acid catalyzed condensation of ethyl alcohol to give diethyl ether. Make sure the mechanism includes protonation, Sn2, and deprotonation.arrow_forwardPredict the products and mechanisms of the following reactions. When more than oneproduct or mechanism is possible, explain which are most likely. 2-chloro-2-methylbutane heated in ethanolarrow_forward[Rh(PPh3)3Cl] is a precatalyst that can be activated by dissociation of a phosphine ligand to form an active catalyst, B, which is used in the hydrogenation of alkenes. The active catalyst can then undergo oxidative addition in the presence of H2 to form complex C. Propene coordinates to C to form complex D, which then undergoes a 1,2- insertion step to form E. Reductive elimination of propane from E regenerates the active catalyst B. Draw complexes B – E and hence provide a full catalytic cycle, including the activation step, for the hydrogenation of propenearrow_forward
- The following alcohols react with HBr to afford an alkyl bromide. Based on their structures, indicate if the reaction mechanism is likely to be Sn1 or Sn2arrow_forwardThe deuterium kinetic isotope effect (kH2O/kD2O) for the hydrolysis of aspirin is 2.2. What does this tell you about the kind of catalysis exerted by the ortho-carboxyl substituent? (Hint: It is easier to break an O¬H bond than an O¬D bond.)arrow_forwardWhat will be the resulting organic products’ structure when the following reactions occur and determine the mechanism (SN1, SN2, E1 or E2)? 1-bromobutane reacts with Potassium Hydroxidearrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

