Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter13: Alcohols, Phenols, And Ethers
Section: Chapter Questions
Problem 13.15E: Classify the following alcohols as primary, secondary, or tertiary: a. b. CH3CH2CH2CH2OH c.
Related questions
Question
 are the following alcohols primary, secondary, or tertiary structures ?
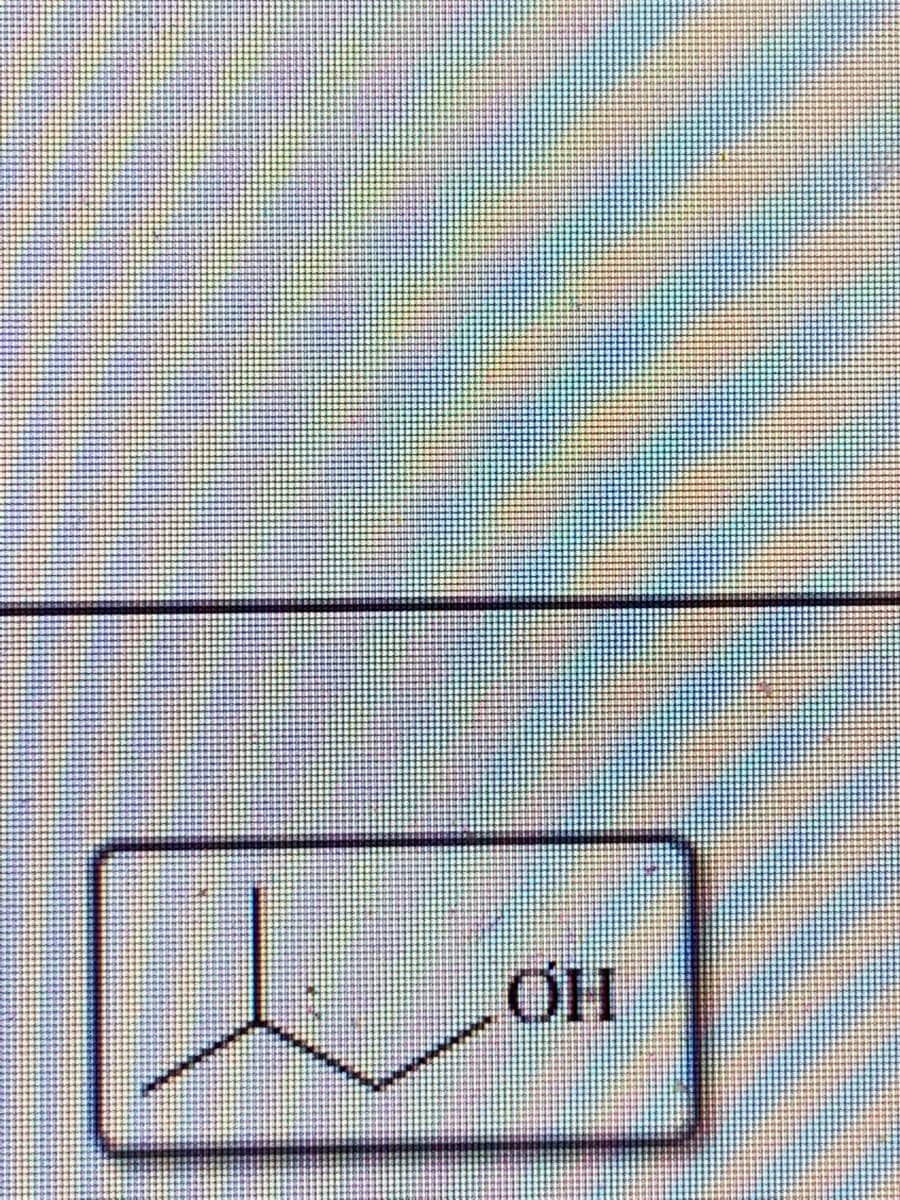
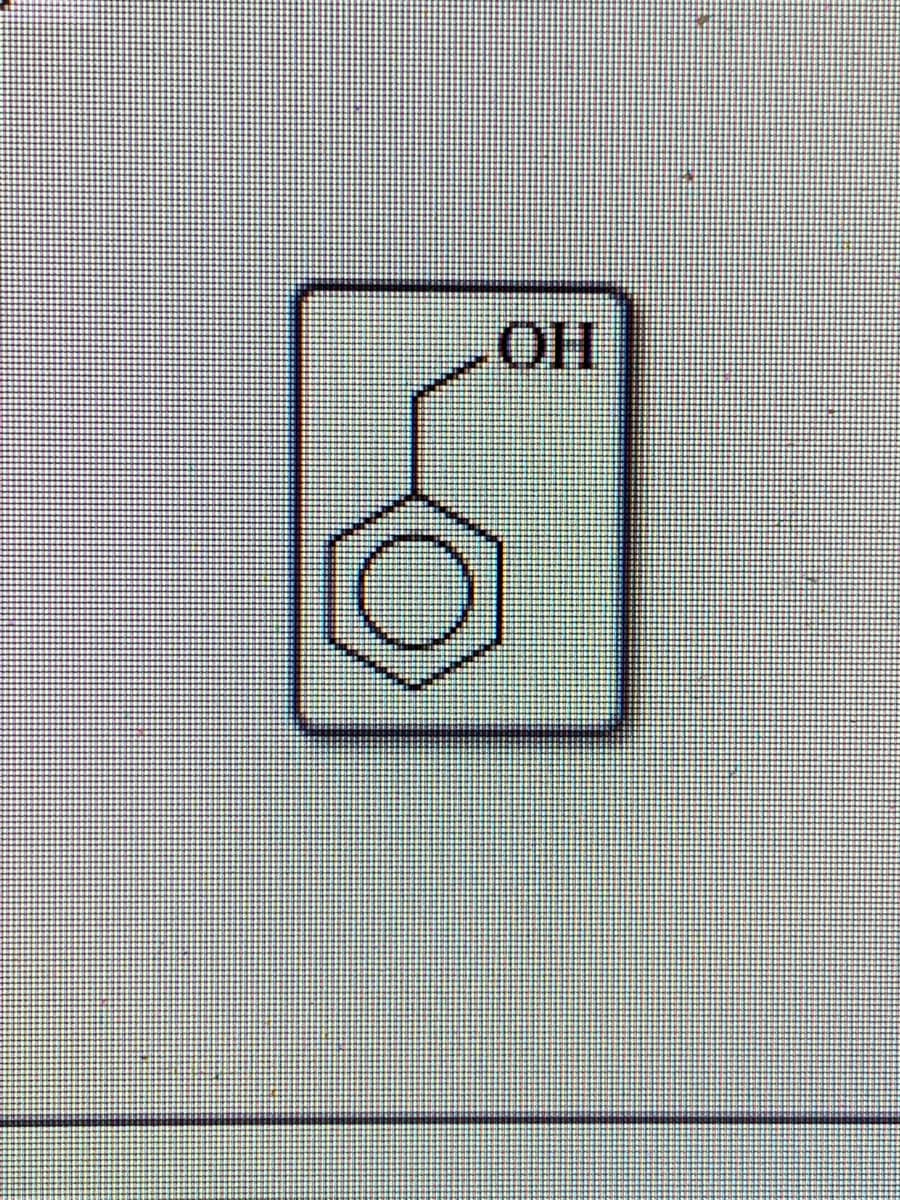
Expert Solution
Step 1
Primary Alcohols
It is an alcohol in which hydroxyl carbon(carbon at which OH group is attached) has a single R group.
Secondary Alcohols
It is an alcohol in which hydroxyl carbon is attached to two R groups.
Tertiary Alcohols
It is an alcohol in which hydroxyl carbon is attached to three R groups.
here R group is the alkyl chain or any carbon containing group.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning