
General, Organic, and Biological Chemistry - 4th edition
4th Edition
ISBN: 9781259883989
Author: by Janice Smith
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 20.4, Problem 20.5PP
Interpretation Introduction
(a)
Interpretation:
The product formed when the given aldose is treated with
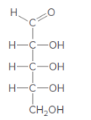
Concept Introduction:
H2 in presence of Pd is a reducing agent and it reduces
Interpretation Introduction
(b)
Interpretation:
The product formed when the given aldose is treated with
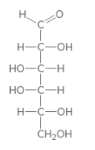
Concept Introduction:
H2 in presence of Pd is a reducing agent and it reduces aldehyde groups into primary alcohols.
Interpretation Introduction
(c)
Interpretation:
The product formed when the given aldose is treated with
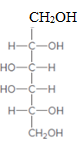
Concept Introduction:
H2 in presence of Pd is a reducing agent and it reduces aldehyde groups into primary alcohols.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
D-Arabinose can exist in both pyranose and furanose forms.a. Draw the a and ß anomers of D-arabinofuranose.b. Draw the a and ß anomers of D-arabinopyranose
What product is formed when a solution of A and B is treated with mild base? This reaction is the rst step in the synthesis of rosuvastatin (sold as a calcium salt under the trade name Crestor), a drug used to treat patients with high cholesterol.
Draw the products formed when A is treated with NBS + hv.
Chapter 20 Solutions
General, Organic, and Biological Chemistry - 4th edition
Ch. 20.1 - Label the hemiacetal carbonthe carbon bonded to...Ch. 20.2 - Prob. 20.1PPCh. 20.2 - Prob. 20.2PCh. 20.2 - Prob. 20.3PCh. 20.2 - Prob. 20.4PCh. 20.2 - Prob. 20.5PCh. 20.2 - Prob. 20.2PPCh. 20.2 - Prob. 20.6PCh. 20.2 - Prob. 20.7PCh. 20.3 - Prob. 20.8P
Ch. 20.3 - Prob. 20.3PPCh. 20.3 - Prob. 20.4PPCh. 20.4 - Prob. 20.5PPCh. 20.4 - Prob. 20.6PPCh. 20.4 - Prob. 20.9PCh. 20.4 - Prob. 20.10PCh. 20.5 - Prob. 20.7PPCh. 20.5 - Prob. 20.8PPCh. 20.5 - Lactose contains both an acetal and a hemiacetal....Ch. 20.5 - Prob. 20.12PCh. 20.5 - Prob. 20.13PCh. 20.5 - Prob. 20.14PCh. 20.5 - Prob. 20.15PCh. 20.6 - Prob. 20.16PCh. 20.6 - Prob. 20.17PCh. 20.7 - Prob. 20.18PCh. 20.7 - Prob. 20.19PCh. 20.8 - Prob. 20.20PCh. 20 - Prob. 21PCh. 20 - Prob. 22PCh. 20 - Prob. 23PCh. 20 - Prob. 24PCh. 20 - Prob. 25PCh. 20 - Prob. 26PCh. 20 - Prob. 27PCh. 20 - Prob. 28PCh. 20 - Prob. 29PCh. 20 - Prob. 30PCh. 20 - Prob. 31PCh. 20 - Prob. 32PCh. 20 - Prob. 33PCh. 20 - Prob. 34PCh. 20 - Prob. 35PCh. 20 - Prob. 36PCh. 20 - Prob. 37PCh. 20 - Prob. 38PCh. 20 - Prob. 39PCh. 20 - Prob. 40PCh. 20 - Prob. 41PCh. 20 - Prob. 42PCh. 20 - Prob. 43PCh. 20 - Prob. 44PCh. 20 - Prob. 45PCh. 20 - Prob. 46PCh. 20 - What product is formed when each compound is...Ch. 20 - What product is formed when each compound is...Ch. 20 - Prob. 49PCh. 20 - Prob. 50PCh. 20 - Prob. 51PCh. 20 - Prob. 52PCh. 20 - Prob. 53PCh. 20 - Prob. 54PCh. 20 - Prob. 55PCh. 20 - Prob. 56PCh. 20 - Prob. 57PCh. 20 - Prob. 58PCh. 20 - Prob. 59PCh. 20 - Prob. 60PCh. 20 - Prob. 61PCh. 20 - Prob. 62PCh. 20 - Prob. 63PCh. 20 - Prob. 64PCh. 20 - Prob. 65PCh. 20 - Prob. 66PCh. 20 - Prob. 67PCh. 20 - Prob. 68PCh. 20 - Prob. 69PCh. 20 - Prob. 70PCh. 20 - Prob. 71PCh. 20 - Prob. 72PCh. 20 - Prob. 73PCh. 20 - Prob. 74PCh. 20 - Prob. 75PCh. 20 - Prob. 76PCh. 20 - Prob. 77PCh. 20 - Prob. 78PCh. 20 - Prob. 79CPCh. 20 - Prob. 80CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The following isomerization reaction, drawn using D-glucose as starting material, occurs with all aldohexoses in the presence of base. Draw a stepwise mechanism that illustrates how each compound is formed.arrow_forwardDraw both pyranose anomers of each aldohexose using a three-dimensional representation with a chair pyranose. Label each anomer as α or β.arrow_forwardDraw the six products (including stereoisomers) formed when A is treated with NBS + hv.arrow_forward
- Draw a stepwise mechanism for the following reaction, the last step in afive-step industrial synthesis of vitamin C that begins with the simplecarbohydrate glucose.arrow_forwardDraw a stepwise mechanism for the conversion of lactone C to carboxylic acid D. C is a key intermediate in the synthesis of prostaglandins by Nobel Laureate E. J. Corey and coworkers at Harvard University.arrow_forwardDraw the organic products for A and B.arrow_forward
- Devise a synthesis of each compound from the given starting material(s). Albuterol is a bronchodilator and proparacaine is a local anesthetic.arrow_forwardDraw a stepwise mechanism for the following isomerization.arrow_forwardDraw all constitutional isomers formed when X is treated with NBS + hv.arrow_forward
- draw the oranic products formed in each reaction. can you answer c, d and farrow_forwardTreatment with sodium borohydride converts aldose A to an optically inactive alditol. Wohl degradation of A forms B, whose alditol is optically inactive. Wohl degradation of B forms d-glyceraldehyde. Identify A and B.arrow_forwardβ-D-Glucose, a hemiacetal, can be converted to a mixture of acetals on treatment with CH3OH in the presence of acid. Draw a stepwise mechanism for this reaction. Explain why two acetals are formed from a single starting material.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY