
Concept explainers
a) cis-1,2-Cyclohexanedicarboxylic acid
Interpretation:
The structure of cis-1,2-cyclohexanedicarboxylic acid is to be given.
Concept introduction:
The names of simple carboxylic acids which are derivatives of open-chain
To give:
The structure of cis-1,2-cyclohexanedicarboxylic acid.
Answer to Problem 32AP
The structure of cis-1,2-cyclohexanedicarboxylic acid is
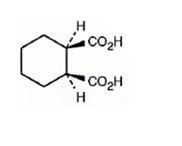
Explanation of Solution
The name shows that the compound has a cyclohexane ring with two carboxylic acid groups in 1,2 positions arranged in the same side of the ring.
The structure of cis-1,2-cyclohexanedicarboxylic acid is

b) Heptanedioic acid
Interpretation:
The structure of heptanedioic acid is to be given.
Concept introduction:
The names of simple carboxylic acids which are derivatives of open-chain alkanes are arrived by replacing the terminal –e of the corresponding alkane name by –oic acid. The numbering starts from carboxyl carbon. Compounds with –COOH bonded to a ring are named using the suffix-carboxylic acid. The –COOH carbon in this case is not numbered as C1, instead the carbon to which it is attached is numbered as C1. As a substituent, the –COOH group is called as carboxyl group.
To give:
The structure of heptanedioic acid.
Answer to Problem 32AP
The structure of heptanedioic acid is
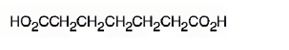
Explanation of Solution
The name shows that the compound has a seven carbon straight chain with two carboxyl groups at theb ends.
The structure of heptanedioic acid is
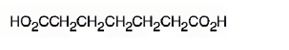
c) 2-Hexen-4-ynoic acid
Interpretation:
The structure of 2-hexen-4-ynoic acid is to be given.
Concept introduction:
The names of simple carboxylic acids which are derivatives of open-chain alkanes are arrived by replacing the terminal –e of the corresponding alkane name by –oic acid. The numbering starts from carboxyl carbon. Compounds with –COOH bonded to a ring are named using the suffix-carboxylic acid. The –COOH carbon in this case is not numbered as C1, instead the carbon to which it is attached is numbered as C1. As a substituent, the –COOH group is called as carboxyl group.
To give:
The structure of 2-hexen-4-ynoic acid.
Answer to Problem 32AP
The structure of 2-hexen-4-ynoic acid is
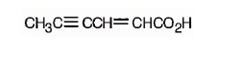
Explanation of Solution
The name shows that the compound has a six carbon straight chain with a carboxylic group, a double bond between C2 & C3 and a triple bond between C4 & C5.
The structure of 2-hexen-4-ynoic acid is
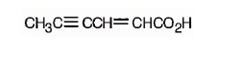
d) 4-Ethyl-2-propyloctanoic acid
Interpretation:
The structure of 4-ethyl-2-propyloctanoic acid is to be given.
Concept introduction:
The names of simple carboxylic acids which are derivatives of open-chain alkanes are arrived by replacing the terminal –e of the corresponding alkane name by –oic acid. The numbering starts from carboxyl carbon. Compounds with –COOH bonded to a ring are named using the suffix-carboxylic acid. The –COOH carbon in this case is not numbered as C1, instead the carbon to which it is attached is numbered as C1. As a substituent, the –COOH group is called as carboxyl group.
To give:
The structure of 4-ethyl-2-propyloctanoic acid.
Answer to Problem 32AP
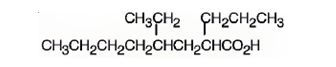
The structure of 4-ethyl-2-propyloctanoic acid is
Explanation of Solution
The name shows that the compound is an octane derivative and has a carboxyl group, a propyl group on C2 and an ethyl group on C4.
The structure of 4-ethyl-2-propyloctanoic acid is

e) 3-Chlorophthalic acid
Interpretation:
The structure of 3-chlorophthalic acid is to be given.
Concept introduction:
The names of simple carboxylic acids which are derivatives of open-chain alkanes are arrived by replacing the terminal –e of the corresponding alkane name by –oic acid. The numbering starts from carboxyl carbon. Compounds with –COOH bonded to a ring are named using the suffix-carboxylic acid. The –COOH carbon in this case is not numbered as C1, instead the carbon to which it is attached is numbered as C1. As a substituent, the –COOH group is called as carboxyl group.
To give:
The structure of 3-chlorophthalic acid.
Answer to Problem 32AP
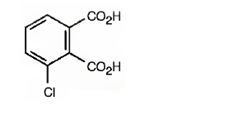
The structure of 3-chlorophthalic acid is
Explanation of Solution
The name indicates that the compound is a benzene derivative with two carboxyl groups on C1 & C2 and a chlorine atom on C3.
The structure of 3-chlorophthalic acid is

f) Triphenylacetic acid
Interpretation:
The structure of triphenylacetic acid is to be given.
Concept introduction:
The names of simple carboxylic acids which are derivatives of open-chain alkanes are arrived by replacing the terminal –e of the corresponding alkane name by –oic acid. The numbering starts from carboxyl carbon. Compounds with –COOH bonded to a ring are named using the suffix-carboxylic acid. The –COOH carbon in this case is not numbered as C1, instead the carbon to which it is attached is numbered as C1. As a substituent, the –COOH group is called as carboxyl group.
To give:
The structure of triphenylacetic acid.
Answer to Problem 32AP
The structure of triphenylacetic acid is
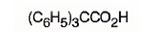
Explanation of Solution
The structure of acetic acid is CH3COOH. The name of the compound given indicates that it has three phenyl groups instead of the three hydrogen atoms present in methyl group in acetic acid.
The structure of triphenylacetic acid is
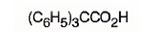
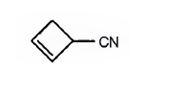
g) 2-Cyclobutenecarbonitrile
Interpretation:
The structure of 2-cyclobutenecarbonitrile is to be given.
Concept introduction:
Simple open chain nitriles are named by adding –nitrile as suffix to the alkane name, with the nitrile carbon numbered as C1. Nitriles can also be names as derivatives of carboxylic acids by replacing the –ic acid or –oic acid ending with –onitrile. The nitrile carbon is not numbered but the carbon to which it is attached is numbered ac C1. If another carboxylic acid derivative is present in the same molecule, the prefix –cyano is used for the –CN group.
To give:
The structure of 2-cyclobutenecarbonitrile.
Answer to Problem 32AP
The structure of 2-cyclobutenecarbonitrile is
Explanation of Solution
The name shows that the compound has a nitrile group attached to a cyclobutene ring with a double bond between C2 & C3.
The structure of 2-cyclobutenecarbonitrile is

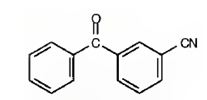
h) m-Benzoylbenzonitrile
Interpretation:
The structure of m-benzoylbenzonitrile is to be given.
Concept introduction:
Simple open chain nitriles are named by adding –nitrile as suffix to the alkane name, with the nitrile carbon numbered as C1. Nitriles can also be names as derivatives of carboxylic acids by replacing the –ic acid or –oic acid ending with –onitrile. The nitrile carbon is not numbered but the carbon to which it is attached is numbered ac C1. If another carboxylic acid derivative is present in the same molecule, the prefix –cyano is used for the –CN group.
To give:
The structure of m-benzoylbenzonitrile.
Answer to Problem 32AP
The structure of m-benzoylbenzonitrile is
Explanation of Solution
The name shows that the compound has a benzene ring attached to a nitrile group and a benzoyl group with meta relationship.
The structure of m-benzoylbenzonitrile is

Want to see more full solutions like this?
Chapter 20 Solutions
Organic Chemistry
- If N,N-diethyl-1-pentanamine reacts with 3-Methylhexanoic acid, draw the resulting skeletal structure and provide its name? Please make sure it shows the answer. It cuts off.arrow_forwardA cyclic, non-aromatic compound containing an attached carboxyl group is named by naming the cyclic compound exclusive of the –COOH group and then adding the suffix - carboxylic acid. Draw the structure of cis-2-methylcyclopentanecarboxylic acid.arrow_forwardDraw structures corresponding to the following: A. 2,3-dimethylhexanoic acid B. 4-methylpentanoic acid C. o-Hydroxybenzoic acid D. trans-Cyclobutane-1, 2-dicarboxylic acidarrow_forward
- Write the structure of the following compounds: a. cyclohexyl propanoate, b. 4-methylheptanonitrile, c. butanoic benzoic anhydride, d. N,N-dibenzylmethamide, e. 3-methylhexanoyl chloride.arrow_forwardIsoerythrogenic acid, C18H26O2, is a acetylic fatty acid that turns vivid vle on exposure to UV light. On Catalytic hydrogenation over a palladium catalyst, five molar equivalents of hydrogen are absorbed, and stearic acidarrow_forwardstructural formula (3E, 6S)-6, 7, 7-trimethyl-4-phenyloct-3-enoic acidarrow_forward
- When the conjugate acid of aniline, C6H5NH3+, reacts with the acetate ion, the following reaction takes place: C6H5NH3+(aq)+CH3COO(aq)C6H5NH2(aq)+CH3COOH(aq) If Kafor C6H5NH3+ is 1.35105 and Kafor CH3COOH is 1.86105 , what is K for the reaction?arrow_forwardHydrolysis of the ester CH3-CH2-COO-CH2-CH2-CH3 in dilute acid, gives a. acetic acid and ethanol b. propanoic acid and ethanol c. propanoic acid and 1- propanol d. acetic acid and 1-propanolarrow_forwardWrite a condensed structural formula for each of the following:(a) an acid with the formula C4H8O2, (b) a cyclicketone with the formula C5H8O, (c) a dihydroxy compoundwith the formula C3H8O2, (d) a cyclic ester with theformula C5H8O2.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
